Background
Henoch-Schönlein purpura (HSP) is a systemic vasculitis predominantly occurring in prepubertal children and characterised by a purpuric rash on the lower extremities, arthralgia and abdominal pain. Renal involvement is not uncommon affecting between 20 and 80% of patients [1] and severe nephrotic syndrome or nephritis is frequently associated with significant renal disease, morbidity and mortality. Lung involvement, especially pulmonary haemorrhage, is rare and reported primarily in adolescents and adults and it carries significant mortality [2]. We report a case of an adult with severe HSP complicated by acute renal failure and pulmonary haemorrhage where aggressive supportive management, corticosteroids and cytotoxic therapy with cyclophosphamide provided life-saving treatment. We discuss the association between systemic HSP and lung involvement as well as options for treatment of combined renal and pulmonary vasculitis in HSP.
Case report
A 54-year-old male presented with a sore throat, arthralgia and fever for 1 week. He had an erythematous rash predominantly involving his lower back and legs bilaterally. He was a heavy smoker for 30 years but had no previous medical problems. On examination he was afebrile and the rash was purpuric in nature. Initial blood tests and mid-stream urine (MSU) were unremarkable. A skin biopsy of the rash showed a leukocytoclastic vasculitis but no tissue was sampled for immunofluorescence. Blood cultures and other serum investigations including anti-nuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (ANCA) and Hepatitis B and C serology were all negative. He was commenced on a non-steroidal anti-inflammatory medication.
One week later, he re-presented with severe colicky abdominal pain with associated nausea and vomiting. He was hypertensive but hypovolaemic and the lower limb rash was still present with moderate peripheral oedema bilaterally. Abdominal X-rays were consistent with a small bowel obstruction and serum creatinine (Cr) was 180 μmol/L (previously 100 μmol/L). MSU demonstrated >1000 × 106/L glomerular red blood cells and a 24-h urine collection 3.6 g/day proteinuria. Repeat vasculitis serology was negative. The management involved intravenous (IV) rehydration, analgesia and naso-gastric tube insertion. A renal biopsy revealed a segmental necrotising glomerulonephritis with small cellular crescents and associated diffuse endocapillary proliferation (Figure 1). Direct immunofluorescence microscopy showed strong granular mesangial and peripheral capillary wall staining for IgA and C3. Electron microscopy showed scattered mesangial, subendothelial and occasional subepithelial ‘hump-like’ deposits.
Fig. 1.
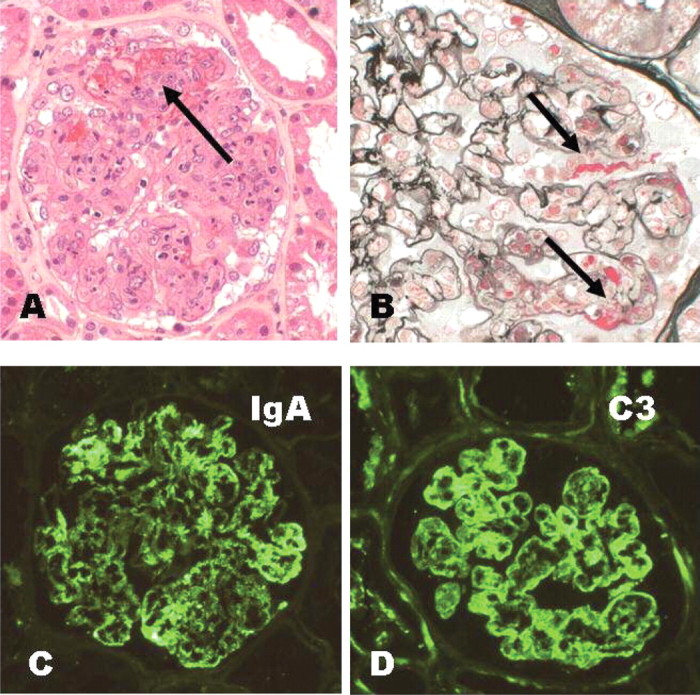
Renal biopsy. (A) The glomerulus shows segmental necrosis with fibrin exudation and a small cellular crescent (arrow). Note also endocapillary hypercellularity and neutrophil polymorph exudation. Haematoxylin & eosin (H&E). Original magnification ×200. (B) There is fibrin within several capillary loops and Bowman's space (arrows). The remaining capillary loops on the left show mild hypercellularity only. Silver trichrome. Original magnification ×400. Direct immunofluorescence microscopy for IgA (C) and C3 (D) shows strong granular mesangial and peripheral capillary wall staining. Original magnification ×200.
A diagnosis of HSP was made and treatment with IV methylprednisolone initiated. Over the next few days serum Cr increased and he became oliguric despite hydration. On Day 6 post-renal biopsy he became acutely dyspnoeic with severe hypoxia. Chest X-ray (CXR) revealed patchy interstitial and alveolar infiltrates bilaterally. With minimal response to diuretics, acute haemodialysis was commenced for fluid removal. He symptomatically improved and a CXR repeated 2 days later had markedly reduced infiltrates. A chest computed tomography (CT) scan was performed that demonstrated diffuse ground-glass opacity bilaterally consistent with pulmonary haemorrhage (Figure 2). A lung biopsy showed extensive fresh intra-alveolar haemorrhage and fibrin with haemosiderin-laden macrophages (Figure 3). Occasional small arteries showed features of a vasculitis with fibrinoid necrosis within the wall (Figure 3); some also showed superimposed luminal thrombus. No viral cyto- pathic effects were found.
Fig. 2.
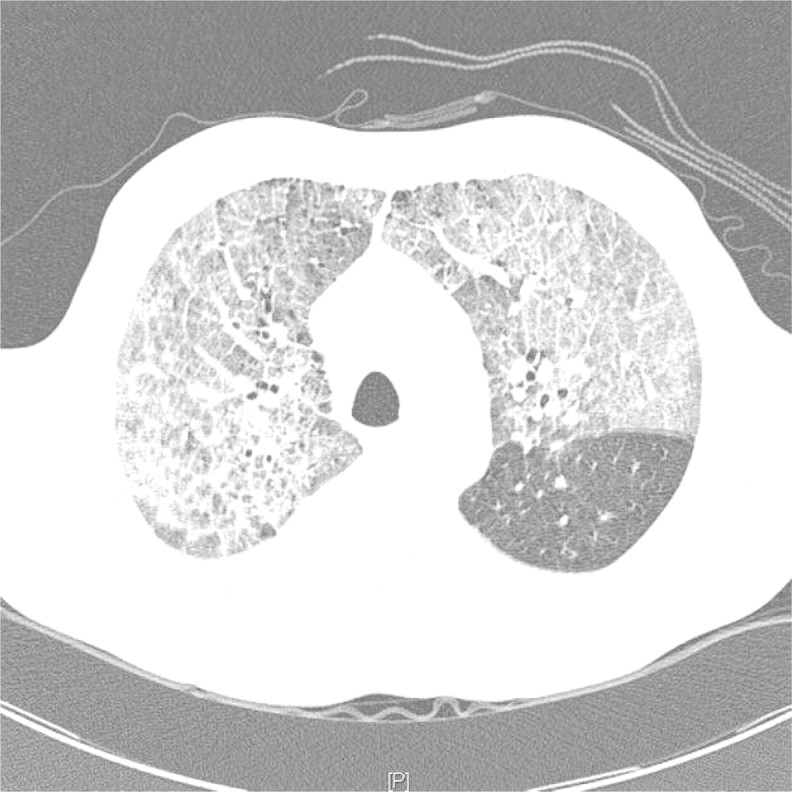
Chest CT. Diffuse ground glass pattern consistent with pulmonary haemorrhage and sparing the left lower lobe.
Fig. 3.
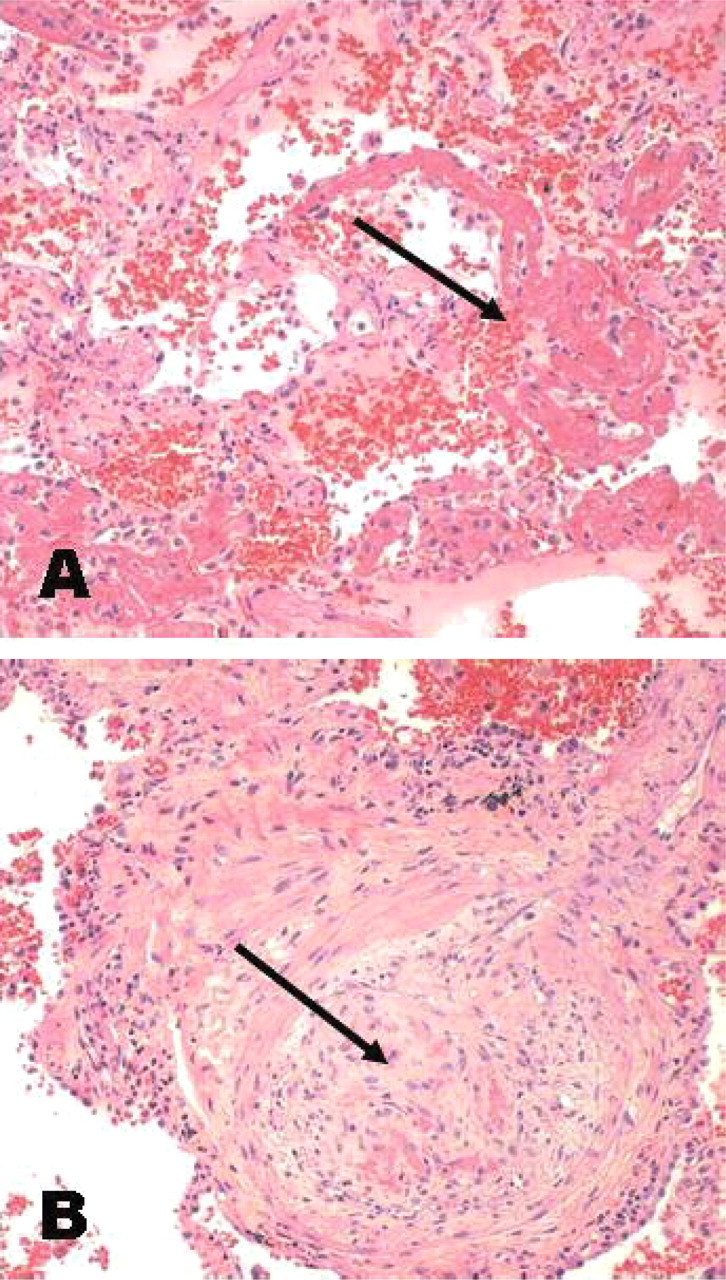
Lung biopsy. (A) There is fresh intra-alveolar haemorrhage and fibrin exudation (arrow). (B) This small artery shows fibrinoid necrosis and intimal proliferation with liminal occlusion (arrow). H&E. Original magnification ×100.
Oral cyclophosphamide was started (2 mg/kg/day) with a further 3 days of IV methylprednisolone. Blood transfusion was given and intermittent haemodialysis was continued. After 3 weeks of cytotoxic therapy, renal function improved and dialysis was ceased with a stable serum Cr 350 μmol/L. Immunosuppression and anti-hypertensive treatment were continued. He was discharged home with portable O2, required for a further 2 months. Three years after initial presentation, his renal function remained stable with serum Cr 200 μmol/L and he had good exertional tolerance. Immunosuppression included prednisolone (5 mg daily) and mycophenolate mofetil, the latter started after 4 months of cyclophosphamide was completed.
Discussion
HSP is a generalized small-vessel vasculitis with a predominant cutaneous component that mainly occurs in children. Adults with this disease are usually more severely affected, more resistant to treatment and usually have a worse prognosis. The classic tetrad of clinical features includes purpuric rash, abdominal pain, arthralgia and renal disease. Clinically overt renal disease occurs in 20–80% of patients with HSP and is not predictably related to the severity of extra-renal manifestations. Renal failure only occurs in ∼1–2% of all patients [1]. Severe renal failure is the main cause of death in fatal cases of HSP and a renal biopsy is recommended where renal impairment is involved, as the severity of histologic lesions is the best indicator of prognosis. Clinically important pulmonary disease is extremely rare in HSP and often fatal [3–6]. We report the case of an adult with severe HSP involving the renal and respiratory systems with successful treatment. We present this case because, despite its rare occurrence, with multiple poor prognostic factors mortality is high and there are few reports of success.
Lung involvement in HSP can be subclinical, is not uncommon in children and can result in interstitial lung disease with slight radiological signs and impaired gas exchange [7,8]. Pulmonary haemorrhage with respiratory failure is rare and reported cases of HSP with pulmonary vasculitis and haemorrhage are few [3–6,9–13]. Diffuse alveolar haemorrhage is associated with significant morbidity and mortality and is more common in adolescents and adults. It usually presents with haemoptysis and a lung biopsy is often required for a diagnosis of pulmonary vasculitis, although the immunofluorescence is often negative for IgA as in our case. Clinical symptoms of respiratory involvement in reported HSP cases include tachypnoea, dyspnoea, chest pain and anaemia.
Lung involvement in primary vasculitis is much more common in Wegener's granulomatosis, Churg-Strauss syndrome and microscopic polyarteritis. A retrospective study of 124 patients with HSP at the Mayo Clinic, Rochester, MN, USA, revealed that only three (2.4%) patients, all adults, had pulmonary involvement [9]. Another study reported impairment in the transfer factor of the lung for carbon monoxide in 28 of 29 children with HSP who were free of pulmonary symptoms, although there were radiological signs of interstitial lung disease in 70% of this population [7]. A prospective study in 15 children addressed a possible relationship between the detection of early lung function abnormalities and alveolar haemorrhage in HSP [8]. Although there were findings of early subclinical lung impairment during the active phase of disease, there was no significant association between pulmonary function abnormalities and the subsequent development of lung haemorrhage. Therefore, impairment in lung function should not be regarded as a sign of progressive lung disease.
Previously reported treatments of pulmonary vasculitis in HSP have included steroids, azathioprine and cyclophosphamide [6,10–12]. Steroids alone are probably not effective in severe cases, and a combination of IV methylprednisolone and cyclophosphamide or azathioprine may achieve better results [6,10]. Although no randomised controlled trials have been performed, cyclophosphamide appears to be the agent of choice in the treatment of this condition. A recent case report described the use of IV cyclosporin A with resolution of pulmonary haemorrhage in a child with HSP [13].
Optimal treatment of severe renal involvement alone is also still unknown. More advanced renal disease, with crescentic nephritis, may respond to more aggressive therapy, and various studies have addressed the use of corticosteroids, cyclophosphamide, azathioprine and cyclosporin A as potential management options [14–16]. However, because spontaneous recovery can often be observed in patients with crescent formation, it is unclear whether these therapeutic regimens are superior to no therapy.
Although no specific conclusion can be made about the appropriate therapy for combined renal and pulmonary vasculitis in HSP, cytotoxic treatment with cyclophosphamide and combined steroids appears to be the most effective management option. The patient in our case presented several poor prognostic factors, including acute renal failure requiring dialysis, nephrotic syndrome, hypertension and pulmonary haemorrhage, and then responded to the treatment with cyclophosphamide and prednisolone achieving pulmonary recovery and becoming dialysis independent.
Conflict of interest statement. None declared.
References
- 1.Saulsbury FT. Henoch-Schönlein purpura in children: report of 100 patients and review of the literature. Medicine. 1999;78:395–409. doi: 10.1097/00005792-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Vats KR, Vats A, Kim Y, et al. Henoch-Schönlein purpura and pulmonary haemorrhage: a report and literature review. Pediatr Nephrol. 1999;13:530–534. doi: 10.1007/s004670050652. [DOI] [PubMed] [Google Scholar]
- 3.Kathuria S, Cheifec G. Fatal pulmonary Henoch-Schönlein purpura syndrome. Chest. 1982;82:654–656. doi: 10.1378/chest.82.5.654. [DOI] [PubMed] [Google Scholar]
- 4.Payton CD, Allison ME, Boutlon-Jones JM. Henoch-Schönlein purpura presenting with pulmonary haemorrhage. Scott Med J. 1987;32:26–27. doi: 10.1177/003693308703200113. [DOI] [PubMed] [Google Scholar]
- 5.Markus HS, Clark JV. Pulmonary haemorrhage in Henoch-Schönlein purpura. Thorax. 1989;44:525–526. doi: 10.1136/thx.44.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson JC, Kelly KJ, Pan CG, et al. Pulmonary disease and hemorrhage in Henoch-Schönlein purpura. Pediatrics. 1992;89:1177–1181. [PubMed] [Google Scholar]
- 7.Chaussain M, Boissieu D de, Kalifa G, et al. Impairment of lung diffusion capacity in Schönlein-Henoch purpura. J Pediatr. 1992;121:12–16. doi: 10.1016/s0022-3476(05)82533-8. [DOI] [PubMed] [Google Scholar]
- 8.Cazzato S, Bernerdi F, Cinti C, et al. Pulmonary function abnormalities in children with Henoch-Schönlein purpura. Eur Respir J. 1999;13:597–601. doi: 10.1183/09031936.99.13359799. [DOI] [PubMed] [Google Scholar]
- 9.Nadrous HF, Yu AC, Specks U, et al. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc. 2004;79:1151–1157. doi: 10.4065/79.9.1151. [DOI] [PubMed] [Google Scholar]
- 10.Al-Harbi NN. Henoch-Schönlein nephritis complicated with pulmonary haemorrhage but treated successfully. Pediatr Nephrol. 2002;17:762–764. doi: 10.1007/s00467-002-0926-y. [DOI] [PubMed] [Google Scholar]
- 11.Kalyoncu M, Cakir M, Erduran E, et al. Henoch-Schönlein purpura: a case with atypical presentation. Rheumatol Int. 2006;26:669–671. doi: 10.1007/s00296-005-0032-0. [DOI] [PubMed] [Google Scholar]
- 12.Tiryaki O, Buyukhatipoglu H, Karakok M, et al. Successful treatment of a rare complication of Henoch-Schönlein purpura in advanced age: pulmonary hemorrhage. Intern Med. 2007:905–907. doi: 10.2169/internalmedicine.46.6272. [DOI] [PubMed] [Google Scholar]
- 13.Matsubayashi R, Matsubayashi T, Fujita N, et al. Pulmonary hemorrhage associated with Henoch-Schönlein purpura. Clin Rheumatol. 2008 doi: 10.1007/s10067-007-0832-6. Jan 15 (Epub) http://www.springerlink.com/content/w05355qg34571567/ [DOI] [PubMed] [Google Scholar]
- 14.Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch-Schöenlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol. 1998;49:9–14. [PubMed] [Google Scholar]
- 15.Tarshish P, Bernstein J, Edelmann CM., Jr Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol. 2004;19:51–56. doi: 10.1007/s00467-003-1315-x. [DOI] [PubMed] [Google Scholar]
- 16.Ronkainen J, Autio-Harmainen H, Nuutinen M. Cyclosporin A for the treatment of severe Henoch-Schönlein glomerulonephritis. Pediatr Nephrol. 2003;18:1138–1142. doi: 10.1007/s00467-003-1245-7. [DOI] [PubMed] [Google Scholar]


