Abstract
We describe the effects of rituximab in two patients with refractory Goodpasture's syndrome. After one single 1000 mg dose administration, rituximab did not appear to improve renal function in either of our cases. Polyoma virus-negative leukoencephalopathy, a probable toxic side effect of rituximab, was seen in our first patient.
Keywords: anti-GBM disease, Goodpasture’s syndrome, leukoencephalopathy, plasma exchange, rituximab
Background
Goodpasture's syndrome is characterized by anti-glomerular basement membrane (GBM) antibodies [1,2] and rapidly progressive glomerulonephritis [3]. The standard therapy of Goodpasture's syndrome consists of immunosuppressive therapy with corticosteroids and cyclophosphamide in combination with plasma exchange, which dramatically improves outcomes [4].
Patients with Goodpasture's syndrome, who are refractory or intolerant to the standard therapy, are still known to have a poor prognosis in terms of mortality and renal survival [3]. Agents that specifically target activated B lymphocytes might provide new treatment of this antibody-mediated autoimmune disorder. Rituximab is a chimeric monoclonal antibody that targets the B lymphocyte antigen CD20 [5]. In one patient with Goodpasture's syndrome, it was possible to discontinue cyclophosphamide and prednisolone after the fourth cycle of rituximab, but it is unclear whether plasmapheresis was continued after administration of rituximab or not [6].
We describe two cases of Goodpasture's syndrome, which were treated with rituximab as rescue therapy, when renal function did not recover with standard treatment.
Case report 1
An 18-year-old woman had been well until 9 months previously. Then, she developed haemoptyses and haematuria. Haemoglobin was 9.4 g/dl, creatinine 2.3 mg/dl, anti-GBM antibodies 57 U/l (normal <10), while c-ANCA, p-ANCA, ANA and ds-DNA antibodies were negative. A renal biopsy was performed, and light microscopy revealed 10 glomeruli, with cellular and partly fibrinoid crescents in all glomeruli. Immunofluorescence showed linear deposition of IgG and C3 at the glomerular basement membrane. Treatment had been started with 100 mg prednisolone per day.
The patient was transferred to our hospital 3 weeks later, when her serum creatinine had risen to 527 μmol/l and urinary protein excretion was 9.17 g/24 h. Physical examination was normal except massive oedema of the legs. Weight was 71 kg, height 181 cm, blood pressure 120/80 mmHg and pulse rate 66/min.
Haemodialysis and daily plasmapheresis (each 4000 ml replaced by 30 g/l albumin) were started 3 days after admission. Additionally, the patient was treated with oral cyclophosphamide 200 mg/day adjusted to leukocyte counts. Prednisolone 100 mg/day was continued for 1 week and then tapered.
Because of the lack of clinical response to this therapy, a single dose of rituximab 1000 mg was administered intravenously on Day 9 after admission. Serum creatinine was 822 μmol/l on Day 14 after 1 week without dialysis. Anti-GBM antibodies could no longer be detected on Day 15 and later. Four weeks after administration of rituximab, CD19/20 cell expression monitored by flow cytometry was negative. In Week 4, plasmapheresis was performed again but renal function did not improve. Haemodialysis had to be resumed in Week 5, persumably because treatment was started in a very late stage of the disease.
In Week 6, the patient had a generalized seizure and had to be admitted to the intensive care unit. MRT of the brain showed enhanced subcortical and pontal signal intensity (Figure 1). The neurological diagnosis was progressive leukoencephalopathy. Examination of the spinal liquor showed no cells and virological examination by PCR disclosed no polyoma virus replication in the liquor. The patient was treated with clobazam and lamotrigine. A second MRT of the brain was performed in Week 10 revealing improvement in the subcortical and pontal signal intensities.
Fig. 1.
Case 1. Cerebral t2-weighted MRI showing a triangular lesion of the corpus callosum with signs of leukoencephalopathy (arrow).
Case report 2
A 20-year-old man who had experienced fatigue for over 2 months was admitted to our hospital with serum creatinine of 3.4 mg/dl and macrohaematuria. Physical examination was normal, weight 88 kg, height 178 cm, heart rate 80 beats/min and blood pressure 124/75 mmHg.
The results of laboratory tests were as follows: haemoglobin 7.8 g/dl, creatinine 370 μmol/l, anti-GBM antibodies 94 kU/l (normal <10), while c-ANCA, p-ANCA, ANA and ds-DNA antibodies were normal. Urine examination showed many red cells with acanthocytes and proteinuria of 1.89 g/g creatinine.
A renal biopsy was performed, and light microscopy showed 8 glomeruli with cellular partly fibrinoid crescents in all of them. Immunofluorescence showed linear deposition of IgG and C3 at the glomerular basement membrane.
Treatment was started with 1000 mg prednisolone intravenously for 3 days and oral cyclophosphamide 200 mg/day. Prednisolone 100 mg/day was given orally for three weeks and then slowly tapered. Sporadic haemodialysis and daily plasmapheresis were started on Day 5.
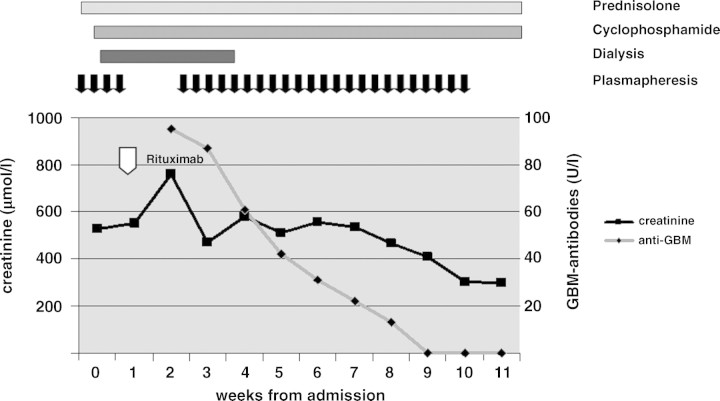
Because of rising creatinine (654 μmol/l), rituximab 1000 mg was administered once intravenously after 2 weeks (Figure 2). Plasmapheresis was ceased in order to prevent inadvertent elimination of rituximab. Four weeks after administration of rituximab, CD19/20 cell cytometry was negative. Since renal function deteriorated, accompanied by oliguria, plasmapheresis was resumed three times a week for 7 weeks. On this therapy, renal function slowly improved. Anti-GBM antibodies could no longer be detected after Week 12 (Figure 2). Renal function recovered and serum creatinine decreased to 300 μmol/l. It was finally possible to discharge the patient from hospital without dialysis after 3 months.
Fig. 2.
Case 2. Levels of serum creatinine and anti-GBM antibodies before and during treatment with cyclophosphamide, prednisolone, plasmapheresis and rituximab (arrow).
Discussion
In this report, we describe the effects of a single dose of rituximab in two patients with refractory Goodpasture's syndrome. Rituximab did not appear to improve renal function in both our cases. Serum creatinine continued to increase after rituximab administration in both cases, but only decreased after plasmapheresis was resumed in case 2. Thus, we are convinced that plasma exchange was more beneficial than rituximab.
It might be speculated that the single rituximab dose (1 × 1000 mg) that was given in our two cases was not enough. But in both patients, CD 19/20 cell count was negative 4 weeks after administration of rituximab. Another case is reported where recurrent anti-GBM disease in a kidney transplant did not respond to rituximab therapy [7].
A severe side effect of rituximab was seen in our first patient caused by JC polyoma virus-negative leukoencephalopathy. The symptoms resolved spontaneously. Recently, cases of JC polyoma virus-positive leukoencephalopathy induced by chemotherapy or rituximab have been described [8]. In our case, leukoencephalopathy was probably a toxic, but not an infectious complication of rituximab.
Conflict of interest statement. None declared.
References
- 1.Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang XP, Fogo AB, Colon S, et al. Distinct epitopes for anti-glomerular basement membrane alport alloantibodies and goodpasture autoantibodies within the noncollagenous domain of alpha3(IV) collagen: a janus-faced antigen. J Am Soc Nephrol. 2005;16:3563–3571. doi: 10.1681/ASN.2005060670. [DOI] [PubMed] [Google Scholar]
- 3.Levy JB, Turner AN, Rees AJ, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–1042. doi: 10.7326/0003-4819-134-11-200106050-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lockwood CM, Boulton-Jones JM, Lowenthal RM, et al. Recovery from Goodpasture's syndrome after immunosuppressive treatment and plasmapheresis. Br Med J. 1975;2:252–254. doi: 10.1136/bmj.2.5965.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzales-Stawinski GV, Yu PB, Parker W, et al. Hapten-induced primary and memory humoral responses are inhibited by the infusion of anti CD20 monoclonal antibody. Clin Immunol. 2001;98:175–179. doi: 10.1006/clim.2000.4980. [DOI] [PubMed] [Google Scholar]
- 6.Arzoo K, Sadeghi S, Liebman HA. Treatment of refractory antibody mediated autoimmune disorders with an anti-CD20 monoclonal antibody (rituximab) Ann Rheum Dis. 2002;61:922–924. doi: 10.1136/ard.61.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauter M, Schmid H, Anders HJ, et al. Loss of a renal graft due to recurrence of anti-GBM disease despite rituximab therapy. Clin Transplant. 2009;23:132–136. doi: 10.1111/j.1399-0012.2008.00912.x. [DOI] [PubMed] [Google Scholar]
- 8.Matteucci P, Magni M, Di Nicola M, et al. Leukoencephalopathy and papovavirus infection after treatment with chemotherapy and anti-CD20 monoclonal antibody. Blood. 2002;100:1104–1115. doi: 10.1182/blood-2002-04-1271. [DOI] [PubMed] [Google Scholar]