Abstract
Bladder malignancy in the kidney transplant recipient is rare and compared with the general population tends to be of high grade and have an aggressive clinical course. In this report, we describe a case of urothelial carcinoma developing in a kidney transplant recipient 6 years after the diagnosis of polyomavirus nephropathy (PVN). BK virus (BKV) DNA was identified in urine and serum by PCR. The diffuse strong staining of SV40 T-antigen and p53 within both the in situ and invasive carcinoma suggest that BKV may play a role in the oncogenic pathway in this clinical setting.
Keywords: BK virus, kidney transplant, PVN, urothelial carcinoma
Introduction
Cancer is second to cardiovascular disease as one of the major causes of morbidity and mortality in kidney transplant recipients [1–3]. An Australian and New Zealand (ANZ) study showed a 3- to 5-fold increase in overall cancer incidence (excluding non-melanoma skin cancer). The risk of developing a neoplasm, skin neoplasms excluded, is ∼15–20% at 10 years, but the rate may rise to 40% at 20 years [2]. Viral-related neoplasia appears to incur the greatest risk when compared with the general population; malignancies include those related to human papillomavirus (HPV) (tongue, mouth, vulva, vagina, penis), Epstein Barr virus (lymphoma), hepatitis B and C (hepatocellular carcinoma) and human herpesvirus 8 (Kaposi's sarcoma). In the ANZ study, there was no excess post-transplantation risk for the two most common cancers in the Australian population, breast and prostate cancer [3]. Bladder malignancy in the kidney transplant recipient is rare with only a limited number of reported cases [2,4–7]. Although the incidence of bladder cancer in organ recipients appears to parallel the general population, the tumours are more likely to be high grade with an aggressive clinical course and poor response to chemotherapy.
Case report
A 46-year-old Asian man with chronic kidney failure due to tuberculosis received a deceased donor kidney transplant in February 2000. He received triple immunosuppressive therapy consisting of tacrolimus, mycophenolate mofetil (MMF) and prednisolone. His subsequent course was uneventful until 2 years post-transplantation (February 2002) when a rise in serum creatinine to 2.1 mg/dL (190 umol/L) prompted a kidney biopsy. The biopsy showed severe polyomavirus nephropathy (PVN) stage B [8], and BK virus (BKV) DNA was identified in urine and serum by PCR. Cyclosporin A and azathioprine were substituted for tacrolimus and MMF. Over the following 6 years, creatinine was stable at 2.1–2.3 mg/dL (190–200 umol/L); BKV DNA remained detectable in the serum at fluctuating low levels of 422–570 copies/mL (2.63–2.76 log10 copies/mL) on the seven occasions when it was measured over these 6 years. Regular urine cytology was performed in the first 2 years following the diagnosis of PVN with no malignant cells seen. In May 2007, urine examination showed a small number of red cells (20 × 106 RBC/L) and in December 2007 urine examination showed 600 × 106 RBC/L. Subsequent urine cytology revealed malignant cells; no definite ‘decoy cells’ were seen. The patient was referred to a urologist, and during cystoscopy urinary bladder biopsies were performed. These showed a high-grade urothelial carcinoma with invasion of the muscularis propria and a focal invasive micropapillary pattern. On immunohistochemistry, the tumour showed diffuse strong nuclear staining for Simian virus 40 (SV40) T-antigen (T-Ag) and P53 in >90% of cells (Figure 1). No normal mucosa was present.
Fig. 1.
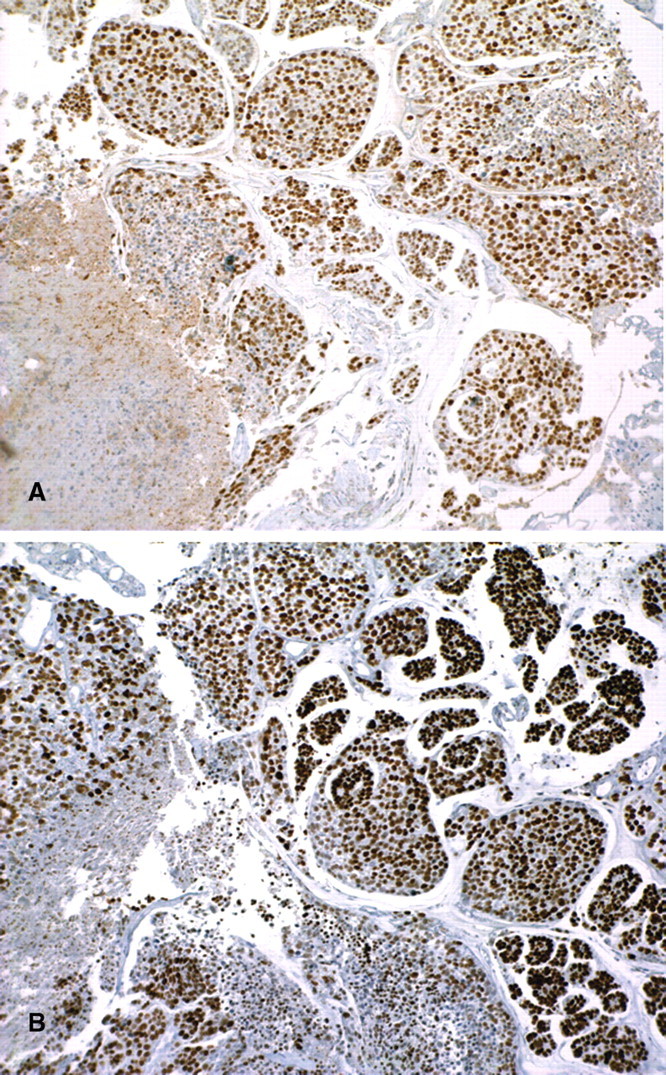
Transurethral biopsy of bladder tumour. Immunohistochemistry shows diffuse strong positive nuclear staining of the invasive high-grade urothelial carcinoma for SV40 T-antigen (A) and p53 (B) (original magnification ×100).
The patient underwent cystectomy with ileal conduit formation and removal of the left native kidney. Within the cystectomy specimen, residual high-grade urothelial carcinoma with invasion into perivesical fat and prominent lymphovascular space invasion was seen (Figure 2). The tumour showed a predominant invasive micropapillary pattern. Elsewhere, the urothelium showed focal evidence of in situ carcinoma (Figure 3). No polyomavirus nuclear changes were evident in the non-malignant urothelium, and there was no convincing cystitis. Diffuse strong expression of SV40 T-Ag was seen in both the invasive and in situ carcinoma (Figure 3), whereas the non-malignant urothelium and atrophic native kidney were negative. P53 also showed diffuse strong nuclear staining in the malignant epithelium in both the in situ and invasive tumour, exactly mirroring the SV40 T-Ag staining (Figure 3).
Fig. 2.
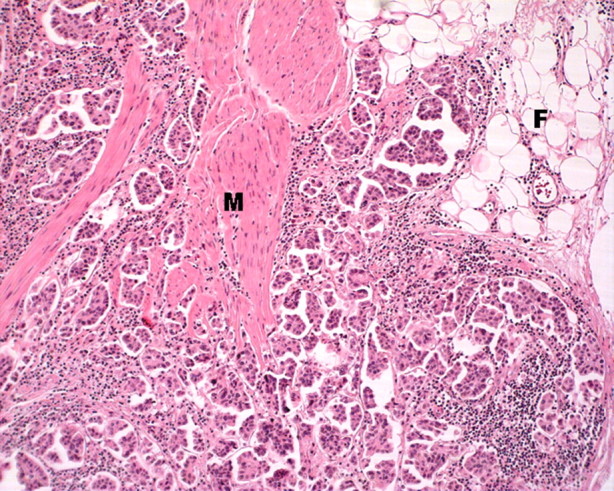
Cystectomy. The high-grade urothelial carcinoma shows a predominant invasive micropapillary pattern with infiltration through the muscularis propria (M) into perivesical fat (F) (haematoxylin and eosin (H&E), original magnification ×100).
Fig. 3.
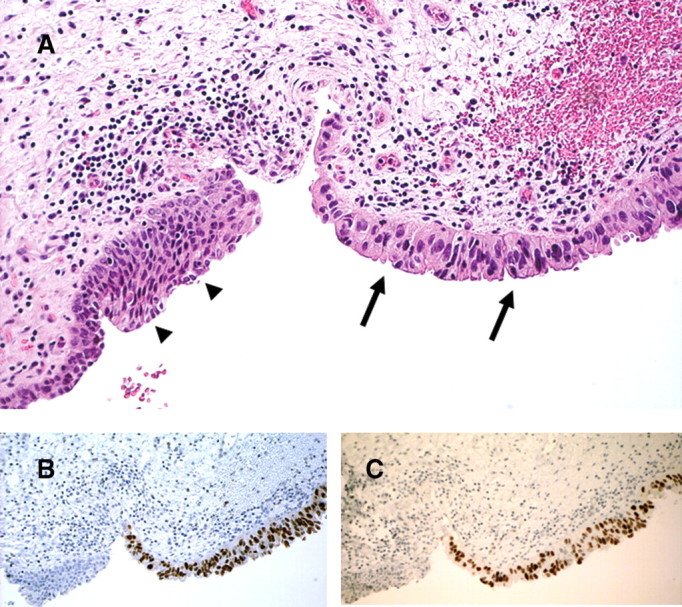
Cystectomy. In situ urothelial carcinoma (arrows) abuts non-malignant urothelium (arrowheads) (H&E). Immunohistochemistry shows strong positive nuclear staining of the in situ carcinoma for both SV40 T-antigen (B) and p53 (C). Note that the non-malignant epithelium is negative (original magnification ×200).
Discussion
Urothelial carcinoma in the general population is more common in men, with male-to-female ratio of 4:1, and has its highest incidence in the sixth and seventh decades. The majority of bladder tumours (∼75%) are low-grade non-invasive urothelial carcinomas that are usually controlled by local resection; however, multiple recurrences are common [5]. In contrast, high-grade urothelial carcinomas are more likely to be invasive and require radical surgery such as complete cystectomy and ileal conduit. Following cystectomy, 5-year survival for tumours invading the muscularis is 40% and for tumours invading perivesical fat, 20% [5]. Arylamines, analgesics, schistosomiasis infection, smoking and cyclophosphamide are known risk factors for bladder cancer by causing prolonged local irritation of the mucosa [5]. In the current case, there was no history of cyclophosphamide therapy.
Compared with the general population, the age of onset of bladder cancers in the transplant recipient is younger (average 44 years) with no male predominance [4–7]. The duration of immunosuppression to development of the urothelial malignancy varies from 2 years to >10 years. The majority of the earlier reported cases had received cyclophosphamide and azathioprine [4,5], but more recent cases have occurred in patients with immunosuppressive regimens including cyclosporine or tacrolimus and MMF [6,7]. The majority of cases are invasive high-grade urothelial carcinoma or squamous carcinoma. This could relate to ongoing immunosuppression and susceptibility to oncogenic viruses.
Studies have investigated possible roles for virus in the aetiology of urothelial carcinoma. Some studies have demonstrated a positive association between HPV and urothelial cancer in humans [9,10]; however, others have failed to confirm this [11]. The potential role of polyomaviruses in bladder cancer has also been investigated in both humans and animal models [6,11,12]. Polyomaviruses code for a non-structural protein, the large T-Ag, which can bind to and inactivate tumour suppressor proteins p53 and pRB, resulting in aberrant cell cycle regulation [12]. Expression of T-Ag induces high-grade bladder tumours in transgenic mice [12]. One study demonstrated a statistically significant association between polyomavirus infection and bladder cancer in a large series of patients [13]. In addition, in an Italian series BKV sequences were reported in 55% of 32 bladder tumours using PCR techniques [11]. However, other studies using identification of polyomavirus DNA have failed to confirm an association of BKV with urothelial carcinoma in the general population [14,15]. A study using tissue microarrays of human bladder tumours and immunohistochemical staining for SV40 T-Ag and p53 showed only very focal staining in occasional tumours [16]. It is important to note that all these studies looked at bladder tumours in immunocompetent individuals.
Polyomaviruses include BKV, JC virus (JCV) and SV40. BKV infects 70% of the human population; primary infection is in childhood and tends to be asymptomatic [17]. After the primary infection, the virus persists in latent form primarily in the kidneys. Immunosuppression triggers viral replication and in some instances disease. BKV is by far the most common polyomavirus that causes kidney disease; JCV and SV40 are rarely implicated [17]. PVN has only emerged as a significant cause of kidney allograft dysfunction in the past 10 years. This is thought to relate to the widespread use of newer more potent immunosuppressive drugs including tacrolimus and MMF. BK viraemia occurs in up to 13% of kidney recipients and PVN in up to 8% [17]. The first case of PVN reported in Australia was published by our group in 2003 [18] and is in fact the same patient we now present with high-grade bladder carcinoma.
Prior to the diagnosis of bladder carcinoma, our patient had a 6-year history of BKV infection who had presented as kidney allograft dysfunction at 2 years post-transplant; kidney transplant biopsy showed PVN stage B. With reduction in immunosuppression, his creatinine remained stable at 2.1 mg/dL (190 umol/L) over several years; BKV DNA remained detectable at low levels in the serum over this period with BKV load 422–570 copies/mL (2.63–2.76 log10 copies/mL). There has been only one previous report of urothelial carcinoma developing in a kidney transplant recipient with a history of PVN [6]. This earlier report showed positive staining of the invasive urothelial carcinoma for SV40 T-Ag and p53, whereas surrounding non-neoplastic urothelium and stromal cells were negative. We have also demonstrated strong expression of SV40 and P53 not only in the invasive tumour but also within in situ urothelial carcinoma in the cystectomy specimen in our patient. This supports the proposal that in situ carcinoma may represent an early stage in the progression to invasive urothelial carcinoma and also suggests that BKV may play a role in the oncogenic pathway in this clinical setting. However, consideration should also be given to the possibility that the virus is demonstrable in the tumour simply because replicating cells provide a better milieu for BKV replication than non-neoplastic cells (BKV depends exclusively on host replication machinery to complete its own life cycle) [19].
The bladder tumour in this case showed a predominantly invasive micropapillary pattern. Micropapillary bladder carcinoma is a rare variant of urothelial carcinoma with a high incidence of lymphovascular space invasion [20,21]. Patients typically have high-stage disease and a poor clinical outcome [20,21]. In the series of Maranchie et al. [20], the micropapillary tumours were p53 negative on immunohistochemistry. This is in contrast to our case that showed strong p53 positivity and perhaps suggests a different molecular pathway of oncogenesis in the setting of BKV infection.
The current case has been treated with radical surgery. The kidney transplant has been preserved and the patient receives ongoing immunosuppression with cyclosporine, azathioprine and prednisolone. Serum creatinine has remained stable over a period of 6 months. The high grade, micropapillary phenotype and advanced stage of the tumour are all poor prognostic indicators.
In conclusion, we report a case of bladder cancer developing 6 years after a diagnosis of PVN in a kidney transplant recipient. PVN has only been recognized as a complication of kidney transplantation in the last 10 years, and time will tell whether it will emerge as a significant risk factor for the subsequent development of bladder cancer in this group. However, in the interim we would recommend close surveillance with urine cytological examination and possibly routine cystoscopy in these patients.
Conflict of interest statement. None declared.
References
- 1.Kyollönen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. 2000;13:S394–S398. doi: 10.1007/s001470050369. [DOI] [PubMed] [Google Scholar]
- 2.Lutz J, Heemann U. Tumours after kidney transplantation. Curr Opin Urol. 2003;13:105–109. doi: 10.1097/00042307-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Vajdic CM, McDonald SP, McCredie MRE, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 4.Lemmers MJ, Barry JM. De novo carcinoma of the lower urinary tract in renal allograft recipients. J Urol. 1990;144:1233–1235. doi: 10.1016/s0022-5347(17)39703-3. [DOI] [PubMed] [Google Scholar]
- 5.Gifford RRM, Wofford JE, Edwards WG. Carcinoma of the bladder in renal transplant patients: a case report and collective review of cases. Transplantation. 1998;12:65–69. [PubMed] [Google Scholar]
- 6.Geetha D, Tong BC, Racusen L, et al. Bladder carcinoma in a transplant recipient: evidence to implicate the BK human polyomavirus as a causal transforming agent. Clin Transplant. 2002;73:1933–1936. doi: 10.1097/00007890-200206270-00015. [DOI] [PubMed] [Google Scholar]
- 7.Zani D, Simeone C, Antonelli A, et al. Cancer in kidney transplantation. Urol Int. 2008;80:329–331. doi: 10.1159/000127352. [DOI] [PubMed] [Google Scholar]
- 8.Drachenberg CB, Hirsch HH, Ramos E, et al. Polyomavirus disease in renal transplantation: review of pathological findings and diagnostic methods. Human Pathol. 2005;36:1245–1255. doi: 10.1016/j.humpath.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Mevorach RA, Cos LR, di Sant’Agnese PA, et al. Human papillomavirus type 6 in grade I transitional cell carcinoma of the urethra. J Urol. 1990;143:126–128. doi: 10.1016/s0022-5347(17)39888-9. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Beltran A, Escudero AL. Human papillomavirus and bladder cancer. Biomed Pharmacother. 1997;51:252–257. doi: 10.1016/s0753-3322(97)83540-8. [DOI] [PubMed] [Google Scholar]
- 11.Fioriti D, Pietropaolo V, Dal Forno S, et al. Urothelial bladder carcinoma and viral infections: different association with human polyomaviruses and papillomaviruses. Int J Immunopathol Pharmacol. 2003;16:283–288. doi: 10.1177/039463200301600315. [DOI] [PubMed] [Google Scholar]
- 12.Grippo PJ, Sandgren EP. Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. Am J Pathol. 2000;157:805–813. doi: 10.1016/S0002-9440(10)64594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinreb DB, Desman GT, Amolat-Apiado MJM, et al. Polyoma virus infection is a prominent risk factor for bladder carcinoma in immunocompetent individuals. Diagn Cytopathol. 2006;34:201–203. doi: 10.1002/dc.20429. [DOI] [PubMed] [Google Scholar]
- 14.Knoll A, Stoehr R, Jilg W, et al. Low frequency of human polyomavirus BKV and JCV DNA in urothelial carcinomas of the renal pelvis and renal cell carcinomas. Oncol Rep. 2003;10:487–491. [PubMed] [Google Scholar]
- 15.Rollison DE, Sexton WJ, Rodriguez AR, et al. Lack of BK virus DNA sequences in most transitional-cell carcinomas of the bladder. Int J Cancer. 2007;120:1248–1251. doi: 10.1002/ijc.22494. [DOI] [PubMed] [Google Scholar]
- 16.Herawi M, Parwani AV, Chan T, et al. Polyoma virus-associated cellular changes in the urine and bladder biopsy samples. Am J Surg Pathol. 2006;30:345–350. doi: 10.1097/01.pas.0000179117.38787.57. [DOI] [PubMed] [Google Scholar]
- 17.Berns JS, Bloom RD. Viral nephropathies. Am J Kidney Dis. 2008;52:370–381. doi: 10.1053/j.ajkd.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Hill PA, Robbie M, Goodman DG, et al. Delayed renal allograft failure due to polyomavirus-associated tubulointerstitial nephritis. Pathology. 2003;35:172–175. [PubMed] [Google Scholar]
- 19.Caracciolo V, Reiss K, Khalili K, et al. Role of the interaction between large T antigen and Rb family members in the oncogenicity of JC virus. Oncogene. 2006;25:5294–5301. doi: 10.1038/sj.onc.1209681. [DOI] [PubMed] [Google Scholar]
- 20.Maranchie JK, Bouyounes BT, Zhang PL, et al. Clinical and pathological characteristics of micropapillary transitional cell carcinoma: a highly aggressive variant. J Urol. 2000;163:748–751. [PubMed] [Google Scholar]
- 21.Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer. Cancer. 2007;110:62–67. doi: 10.1002/cncr.22756. [DOI] [PubMed] [Google Scholar]


