Abstract
Chronic kidney disease (CKD) is a common and costly medical condition, and currently available therapeutic options remain unsatisfactory. Vitamin D analogues are widely used for the bone and mineral disorder associated with CKD. However, accumulating evidence suggests that vitamin D analogues may have actions other than their effects on bone and mineral metabolism. In this article, we review the following aspects on the use of vitamin D analogues for the treatment of CKD: (1) epidemiological studies showing that patients with late-stage CKD have better survival than untreated patients; (2) animal studies showing that vitamin D analogues may retard the progression of CKD; (3) human studies on the anti-proteinuric and possibly renal protecting effects of vitamin D analogues in CKD and (4) the potential mechanisms of its therapeutic benefit. Nonetheless, definitive proof of the clinical benefits by randomized control trial would be necessary before one could advocate the routine use of vitamin D analogues for the treatment of CKD patients.
Keywords: calcitriol, cardiovascular disease, paricalcitol, proteinuria, renal failure
Chronic kidney disease (CKD) is a debilitating and costly medical condition. The clinical course is characterized by persistent proteinuria after an initial insult to the kidney, followed by progressive decline in renal function [1]. CKD patients are not only at risk of progressing to dialysis-dependent renal failure; they are at high risk of developing renal bone disease [2] and cardiovascular disease (CVD) [3,4]—the latter represents the major cause of mortality and morbidity in CKD patients. Unfortunately, the optimal therapy of CKD remains unknown. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) reduced proteinuria in short-term trials [5–7] and retard the rate of progression of renal function deterioration in chronic proteinuric nephropathy [8]. There is also evidence that these therapies may have a beneficial effect on the inflammatory component of CKD [9,10]. However, ACE inhibitor and ARB may not completely abrogate the renal function deterioration among high-risk patients.
In this respect, there is an increasing interest in the use of vitamin D analogues for the treatment of CKD patients. The reduction of renal 1α-hydroxylase activity and the circulating level of 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3), the active form of vitamin D, are prominent features of CKD. Vitamin D is a primary regulator of calcium homeostasis. Deficiency of 1,25-(OH)2D3 plays an important role in the pathogenesis of renal bone disease. Genetic inactivation of either the vitamin D receptor (VDR), a member of the nuclear receptor superfamily that mediates the action of 1,25-(OH)2D3, or 25-hydroxyvitamin D3 1α-hydroxylase, the rate-limiting enzyme for the biosynthesis of 1,25-(OH)2D3, results in impaired calcium homeostasis, leading to hypocalcaemia, secondary hyperparathyroidism and rickets [11–14]. Vitamin D deficiency in adults without CKD can precipitate or exacerbate osteopaenia and osteoporosis, cause osteomalacia and muscle weakness, and increase the risk of fracture [15]. However, the wide tissue distribution of vitamin D receptor (VDR) suggests that the vitamin D endocrine system has additional physiological functions beyond calcium homeostasis. Of great interest is the role it can play in decreasing the risk of many chronic illnesses, including common cancers, autoimmune diseases, infectious diseases and CVD [15], all being relevant in normal population as well as CKD patients.
Vitamin D analogue and mortality in CKD
The use of vitamin D analogues in CKD patients is not new. In fact, replacement of activated vitamin D has been the cornerstone of therapy for secondary hyperparathyroidism, the cardinal manifestation of mineral bone disorder in CKD. While the only current indication for the use of activated vitamin D and its analogues in patients with CKD is the treatment of secondary hyperparathyroidism, the impact of vitamin D therapy may extend beyond lowering parathyroid hormone (PTH) levels and include potential cardiovascular and metabolic benefits. Several large observational studies examining outcomes associated with the use of activated vitamin D therapy in patients on maintenance dialysis and in patients with CKD not yet on dialysis have suggested that the benefits of vitamin D receptor activators may include direct cardiovascular and metabolic benefits (Table 1). These studies incorporated data from a very large number of patients and consistently showed that patients treated with any kind of vitamin D receptor activators experienced significantly lower all-cause and cardiovascular mortality rates compared with patients not receiving any treatment. Furthermore, the survival benefit of vitamin D analogues generally did not change quantitatively after adjusting for serum calcium, phosphate and parathyroid hormone levels in these studies [16–21], suggesting that the effect on survival is almost entirely explained by some non-traditional pleiotropic effects.
Table 1.
Observational studies examining outcomes associated with treatment of activated vitamin D in patients with chronic kidney diseases
| AHR of all-cause | |||||
|---|---|---|---|---|---|
| Study | Patients | Treatment | Follow-up | mortality | Comments |
| Shoji et al. [16] | 242, prevalent HD | Oral alphacalcidol | 61 months | No difference | Lower cardiovascular mortality |
| Teng et al. [17] | 51 037, prevalent HD | Any analogue | 24 months | 0.74 (95% CI 0.71–0.79) | |
| Melamed et al. [18] | 1007, incident HD and PD | Calcitriol | 36 months | 0.62 (95% CI 0.44–0.86) | |
| Kalantar-Zadeh et al. [19] | 58 058, prevalent HD | Paricalcitol | 24 months | Lower all-cause mortality | Possibly a dose-dependent effect |
| Tentori et al. [20] | 7731, prevalent HD | Any analogue | 37 weeks | 0.83 (95% CI 0.77–0.91) | No difference between all three different analogues |
| Teng et al. [21] | 67 399, prevalent HD | Paricalcitol | 36 months | 0.84 (95% CI 0.79–0.90) | Versus calcitriol |
| Kovesdy et al. [22] | 520, CKD stages 2–5 | Calcitriol | 2.1 years | 0.35 (95% CI 0.23–0.54) | Pre-dialysis patientsa |
| Shoben et al. [23] | 1418, CKD stages 3 and 4 | Calcitriol | 1.9 years | 0.74 (95% CI 0.58–0.95) | Pre-dialysis patientsa |
HD, haemodialysis; PD, peritoneal dialysis; CKD, chronic kidney diseases; AHR, adjusted hazard ratio; CI, confidence interval.
aBoth showed a trend of lower risk of dialysis. See the text for detailed description.
It is important to recognize that most of these studies examined CKD patients on maintenance dialysis. Nonetheless, two studies showed a similar benefit in pre-dialysis CKD patients [22,23]. Kovesdy et al. [22] examined the association of oral calcitriol treatment with mortality and the incidence rate of dialysis in 520 male US veterans with CKD stages 3–5 and not yet receiving dialysis; 258 patients received treatment with calcitriol, 0.25–0.5 μg/day, for a median duration of 2.1 years. The incidence rate ratios for mortality and combined death and dialysis initiation were significantly lower in treated patients. In this study, treatment with calcitriol was also associated with a trend towards a lower incidence rate of dialysis. In another study, Shoben et al. [23] evaluated associations of oral calcitriol use with mortality and dialysis dependence in 1418 nondialysis patients with CKD and hyperparathyroidism in the Veterans’ Affairs Consumer Health Information and Performance Sets database. After adjustment for various clinical confounders, oral calcitriol use was associated with a 26% lower risk for death and a 20% lower risk for death or dialysis. Taken together, these two studies suggest that vitamin D analogues may have some renal protective effect in pre-dialysis CKD patients.
Renal protection in animal models
Several studies have shown that the administration of vitamin D analogues attenuates the renal damage in various experimental models of uraemia [24–28] and is summarized in Table 2. Schwarz et al. [24] studied subtotally nephrectomized rats treated with 1,25-(OH)2D3 and found that vitamin D reduces renal cell proliferation and glomerular growth as well as glomerulosclerosis and albuminuria as indicators of progressive glomerular damage. With a similar subtotally nephrectomized rat model, Hirata et al. [25] showed that 22-oxacalcitriol treatment significantly suppressed urinary albumin excretion, prevented increases in serum creatinine and serum urea nitrogen and inhibited glomerular cell number, glomerulosclerosis ratio and glomerular volume. With the same model, Mizobuchi et al. [26] showed that paricalcitol can suppress macrophage infiltration, monocyte chemoattractant protein-1 (MCP-1) production, transforming growth factor-beta-1 (TGF-β1) mRNA and protein expression, and phosphorylation of Smad2, and these effects are amplified when blood pressure is controlled via renin–angiotensin system blockade.
Table 2.
Animal studies examining the renal protective effect of vitamin D analogues
| Benefit | ||||||
|---|---|---|---|---|---|---|
| Renal | ||||||
| Study | Model | Treatment | Proteinuria | function | Histology | Remarks |
| Schwarz et al. [24] | Subtotally nephrectomy | Calcitriol | Reduce | n/a | Less glomerulosclerosis | No change in BP; benefit not related to PTH |
| Hirata et al. [25] | Subtotally nephrectomy | 22-oxacalcitriol | Reduce | n/a | Less glomerulosclerosis | |
| Mizobuchi et al. [26] | Subtotally nephrectomy | Paricalcitol | Reduce | Improve | Less glomerulosclerosis | Possibly a higher TI volume |
| Migliori et al. [27] | Thy1.1 glomerulonephritis | Calcitriol | Reduce | n/a | Less glomerular damage | |
| Tan et al. [28] | Obstructive nephropathy | Paricalcitol | n/a | n/a | Less TI damage | |
BP, blood pressure; PTH, parathyroid hormone; TI, tubulointerstitial; n/a, not assessed.
Glomerular and tubulointerstitium have both been proposed as the target of vitamin D in renal protection. In a study of Thy1.1 experimental glomerulonephritis, Migliori et al. [27] found that treatment with 1,25-(OH)2D3 abrogated podocytes injury, detected as desmin expression, and loss of nephrin and zonula occludens-1 (two slit diaphragm-associated proteins) and glomerular polyanion staining, suggesting that vitamin D may revert proteinuria by counteracting glomerular podocyte injury. On the other hand, in a study of experimental obstructive nephropathy, a model that is characterized by predominant tubulointerstitial lesions, Tan et al. [28] showed that paricalcitol significantly attenuated renal interstitial fibrosis, as demonstrated by a reduced interstitial volume, decreased collagen deposition and repressed mRNA expression of fibronectin and type I and type III collagens. Paricalcitol also preserved E-cadherin and reduced alpha-smooth muscle actin expression in vivo. In addition, paricalcitol suppressed renal TGF-β1 and its type I receptor expression, underscoring its ability to block directly the epithelial to mesenchymal transition of tubular epithelial cells.
Renal protection in human CKD
Despite a wealth in animal data, human study on the renal protective effect of vitamin D analogues is scarce (Table 3). Neither of the observational studies described earlier on the outcomes associated with treatment of activated vitamin D in patients with pre-dialysis CKD provides a conclusive answer [22,23]. In the study reported by Shoben et al. [23], the incidence rate of dialysis was 10.4 and 10.1 events per 100 person-years among calcitriol users and nonusers, respectively. In contrast, Kovesdy et al. [22] found an insignificant trend between calcitriol treatment and the incidence rate of dialysis-dependent renal failure alone (without mortality) in unadjusted and fully adjusted models (adjusted hazard ratio 0.73, 95% CI 0.50–1.12). In fact, the association was statistically significant in the case-mix-adjusted model, with an incidence rate ratio of 0.67 (95% CI 0.46–0.97). Although the result seems encouraging, it is important to note that the study of Kovesdy et al. [22] had a smaller sample size and involved a more heterogeneous group of CKD patients than the one reported by Shoben et al. [23] (see Table 1).
Table 3.
Human studies examining the renal protective effect of vitamin D analogues
| Study | Type | Patients | Treatment | Follow-up | Proteinuria | Renal function | Comments |
|---|---|---|---|---|---|---|---|
| Kovesdy et al. [22] | Cohort | 520, CKD stages 2–5 | Calcitriol | 2.1 years | n/a | Incidence rate ratio of dialysis 0.67 (95% CI, 0.46 to 0.97) | |
| Shoben et al. [23] | Cohort | 1418, CKD stages 3 and 4 | Calcitriol | 1.9 years | n/a | No difference in the incidence rate of dialysis | |
| Agarwal et al. [29] | Prospective | 220, CKD stages 3 and 4 | Paricalcitol | 24 weeks | Reduced (OR 3.2, 95% CI 1.5–6.9) | n/a | Proteinuria tested by dipstick only |
| Szeto et al. [30] | Prospective | 10, IgA nephropathy | Calcitriol | 12 weeks | Reduced (1.98 ± 0.74 to 1.48 ± 0.81 g:g-Cr) | No difference before and after treatment | No difference in the serum AgII level |
| Alborzi et al. [31] | Prospective | 24, CKD stages 2 and 3 | Paricalcitol | 1 month | Reduced by 46% (95% CI, 17–65%) | No difference between treatment and placebo groups | Reduction in serum CRP |
CKD, chronic kidney diseases; AgII, angiotensin II; CRP, C-reactive protein; CI, confidence interval; OR, odds ratio; n/a, not assessed.
How about prospective studies? In three double-blind, randomized, placebo-controlled studies to evaluate the safety and efficacy of oral paricalcitol, Agarwal et al. [29] studied 220 CKD stage 3 and 4 patients with secondary hyperparathyroidism who were randomized to oral paricalcitol (mean dose 9.5 μg/week) or placebo and followed up for up to 24 weeks. At the final visit, 51% of the paricalcitol patients compared to 25% placebo patients had a reduction in proteinuria (odds for the reduction in proteinuria 3.2 times greater for paricalcitol patients, 95% CI 1.5–6.9). The reduction of proteinuria favoured patients on paricalcitol regardless of the use of therapies to block the renin–angiotensin–aldosterone system. However, the actual amount of proteinuria, which was not the primary objective of those original trials, was not fully quantified. It could also be argued that the qualitative difference in proteinuria may be mediated by the difference in the achieved parathyroid hormone level between groups, for which the study was designed.
Two prospective human studies specifically examined the renal protective effect of vitamin D analogues. In a recent open-label prospective uncontrolled trial of 10 patients with biopsy-proven IgA nephropathy and persistent proteinuria despite ACE inhibitor or ARB therapy, we found that calcitriol treatment, 0.5 μg twice weekly for 12 weeks, resulted in a progressive decrease in urine protein–creatinine ratio during the first 6 weeks that persisted throughout the study period [30]. This study, however, did not have a control group, and the duration of follow-up was too short to access the benefit on renal function deterioration. In this study, there was a simultaneous decrease in the serum TGF-β level, and the percentage of decrease in the serum TGF-β level significantly correlated with the percentage of change in proteinuria. The serum angiotensin II level, however, did not change throughout the study, indicating that the anti-proteinuric effect of calcitriol may not be related to the effect on the renin–angiotensin system.
In another recent pilot trial, 24 CKD patients (mostly stage 3) were randomly allocated to receive 0, 1 or 2 μg oral paricalcitol [31]. After 1 month, the treatment-to-baseline ratio of 24-h albumin excretion rate was 1.35 (95% CI, 1.08–1.69) with placebo, 0.52 (95% CI, 0.40–0.69) with a 1 μg dose and 0.54 (95% CI, 0.35–0.83) with a 2 μg dose. In addition, there was a significant reduction in the serum C-reactive protein level in the treatment groups. In this study, no difference was observed in renal function, 24-h ambulatory blood pressure or parathyroid hormone between groups. It was concluded that paricalcitol-induced reduction in albuminuria and inflammation may be mediated independent of its effects on haemodynamics or parathyroid hormone suppression. Taken together, these two studies showed an anti-proteinuric effect of vitamin D analogues by short-term treatment. Since both of the studies had very short follow-up, the long-term effect on renal function deterioration remains undetermined.
Diabetic nephropathy: a special case?
The above discussion considered CKD, irrespective of the underlying renal diagnosis, as a whole group. As to the use of vitamin D analogues, diabetic nephropathy needs special considerations because it is the leading cause of dialysis-dependent end-stage renal disease, and the renin–angiotensin system (RAS) is a major mediator of progressive renal injury in diabetic nephropathy. As discussed above, vitamin D negatively regulates the RAS, and the renal protective role of vitamin D in diabetic nephropathy has been specifically studied [32]. Diabetic VDR knockout mice developed more severe albuminuria and glomerulosclerosis due to increased glomerular basement membrane thickening and podocyte effacement [33]. More fibronectin and less nephrin were expressed in the VDR knockout mice compared to diabetic wild-type mice. In receptor knockout mice, increased renin, angiotensinogen, TGF-β and connective tissue growth factor accompanied the more severe renal injury.
In cell culture models, Zhang et al. [33] showed that 1,25-(OH)2D3 inhibited high glucose-induced fibronectin production in cultured mesangial cells and increased nephrin expression in cultured podocytes. The 1,25-(OH)2D3 also suppressed high glucose-induced activation of the renin–angiotensin system and TGF-β in mesangial and juxtaglomerular cells. In another series of experiments, the same group of investigators showed that combination therapy with an ARB and a vitamin D analogue markedly ameliorated renal injury in the streptozotocin-induced diabetes model due to the blockade of the compensatory renin rise by the vitamin D analogue, leading to more effective inhibition of the renin–angiotensin system [34]. The combined treatment suppressed the induction of fibronection, TGF-β and MCP-1 and reversed the decline of slit diaphragm proteins nephrin, zonula occludens-1 and alpha-actinin-4. These were accompanied by blockade of intrarenal renin and angiotensin II accumulation induced by hyperglycaemia and losartan. Despite the above-mentioned experimental evidence, however, there are no human data in this area, and it seems probable that the renal protective effect of vitamin D is not specific for diabetic nephropathy [35].
Mechanisms of vitamin D-related benefits
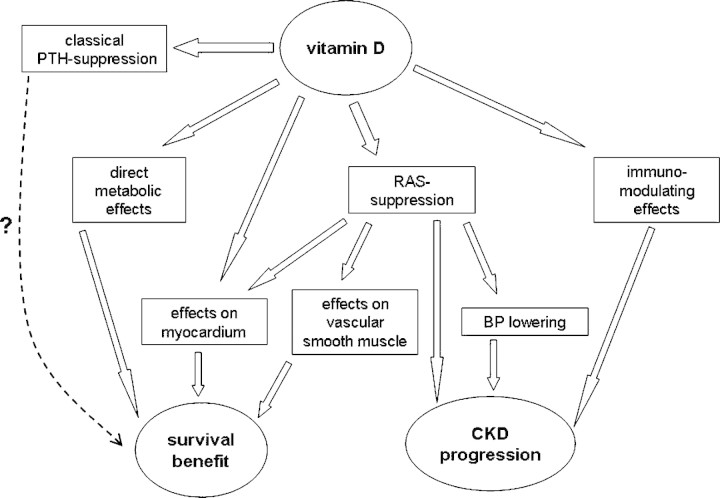
A number of possible mechanisms have been proposed to explain the survival advantage of CKD patients treated with vitamin D analogues [36] (Figure 1). In addition to its primary role in calcium homeostasis and bone mineralization, the vitamin D endocrine system has additional physiological functions involving the immune and cardiovascular system and the protection of renal cellular integrity [37,38]. Most of the pleiotropic actions of vitamin D and its analogues are mediated by the VDR, a ligand-dependent transcription factor belonging to the steroid nuclear receptor gene family [38].
Fig. 1.
Summary of putative mechanisms of action responsible for the lower mortality and progression of chronic kidney disease associated with vitamin D therapy. BP, blood pressure; CKD, chronic kidney diseases; RAS, renin–angiotensin system.
Effects on blood pressure and the renin–angiotensin system
In the last two decades, epidemiological studies have revealed an inverse relationship between the plasma 1,25-(OH)2D3 concentration and the blood pressure or the plasma renin activity in both normotensive men and patients with essential hypertension [39–43]. Ultraviolet light exposure, which is required for vitamin D biosynthesis, is inversely related to the rise of blood pressure and the prevalence of hypertension in the general population and was shown to have blood pressure-lowering effects [44,45]. Furthermore, it has been reported that vitamin D3 supplementation reduces blood pressure in patients with essential hypertension [46,47], and 1,25-(OH)2D3 treatment reduces blood pressure, plasma renin activity and Ang II levels in hyperparathyroidism patients [48,49].
Experimental studies have highlighted the role of the VDR as a down-regulator of the renin–angiotensin system [50,51]. Li et al. [50] show that renin expression and plasma angiotensin II production were increased several folds in vitamin D receptor-null mice, leading to hypertension, cardiac hypertrophy and increased water intake. In wild-type mice, inhibition of 1,25-(OH)2D3 synthesis also led to an increase in renin expression, whereas 1,25-(OH)2D3 injection led to renin suppression. In this experiment, vitamin D regulation of renin expression was independent of calcium metabolism and that 1,25-(OH)2D3 markedly suppressed renin transcription by a VDR-mediated mechanism in cell cultures. Similarly, Zhou et al. [52] showed that in 1α-hydroxylase knockout mice with a rescue diet that normalizes serum calcium and phorphorus levels, abnormalities in the renin–angiotensin system, blood pressure and cardiac structure–function problems remained, indicating that the repressing effect of vitamin D on the renin–angiotensin system is independent of extracellular calcium or phosphorus. Mechanistic analysis of VDR-mediated renin suppression suggests that VDR-mediated renin suppression likely acts through a transcriptional regulatory complex that binds to the CRE-like domain in the renin enhancer region [53].
The effect of a vitamin D analogue on the renin–angiotensin system, however, may not be directed on renin alone [54]. In rats with the remnant kidney model of chronic renal failure (5/6 nephrectomy), Freundlich et al. [55] showed that paricalcitol decreases angiotensinogen, renin, renin receptor and vascular endothelial growth factor mRNA levels in the remnant kidney by 30–50% as compared to untreated animals. Similarly, the protein expression of renin, renin receptor, the angiotensin type 1 receptor and vascular endothelial growth factor were all significantly decreased.
Other cardiovascular effects
Vitamin D has a direct effect on myocardium, independent of its suppressive effect on the renin–angiotensin system. It directly suppresses myocardial hypertrophy and could improve cardiac performance [56]. Walters et al. [57] demonstrated that the uptake of calcium by cardiac muscle cells is in part regulated by vitamin D, an effect that is receptor mediated, requiring transcription and protein synthesis. Vitamin D has also been shown to affect the growth of cardiac cells in culture. O’Connell et al. [58] showed that 1,25-(OH)2D3 reduces proliferation rate, PCNA levels and c-Myc levels of myocytes in culture through decreased entry into the S phase. Vitamin D deficiency also results in a shift in the tissue distribution of V1 and V3 myosin chains, favouring the V1 isotype [59]. Shifts in myosin isotypes have been shown to alter myocyte contractility [60].
In addition, vitamin D has a biphasic action on vascular smooth muscle cell and prevents its proliferation [61]. Carthy et al. [62] found that 1,25-(OH)2D3 suppresses VS vascular smooth muscle cell 3H-thymidine uptake; active vitamin D also suppresses the stimulatory effect of epidermal growth factor on vascular smooth muscle cell proliferation. At low dose, vitamin D may also stimulate the production of inhibitors of arterial calcification [63,64] and have an anti-thrombotic effect [65]. In contrast, active form of vitamin D applied in supraphysiological concentration of 10 nmol/l is a mitogenic factor for aortal smooth muscle cell [66].
Other metabolic effects
Vitamin D has also been reported to enhance insulin sensitivity and induce a favourable plasma lipid profile. In 126 healthy, glucose-tolerant subjects, Chiu et al. [67] found an independent correlation between serum 25(OH)D concentration and insulin sensitivity index, and an independent negative relationship of 25(OH)D concentration with plasma glucose concentration at fasting, 60, 90 and 120 min during the oral glucose tolerance test. In this study, subjects with hypovitaminosis D (below 20 ng/mL) had a greater prevalence of components of metabolic syndrome than did subjects without hypovitaminosis D. Vitamin D has also been reported to increase apolipoprotein A-I and high-density lipoprotein (HDL) cholesterol, resulting in a favourable plasma lipid profile [68]. In HepG2 cells treated 1,25-(OH)2D3, Apo AI secretion and mRNA levels were both suppressed in a dose-dependent manner [69]. This was accompanied by a similar decrease in apo AI promoter activity.
Immunomodulating effects
A vitamin D analogue is also a potent regulator of the immune system, in particular, on T cell-medicated immunity [70,71]. Vitamin D receptor is found in significant concentrations in the T lymphocyte and macrophage populations. However, its highest concentration is in the immature immune cells of the thymus and the mature CD8 T lymphocytes. The significant role of vitamin D compounds as selective immunosuppressants is illustrated by their ability to either prevent or markedly suppress animal models of autoimmune disease. Results show that 1,25-(OH)2D3 can either prevent or markedly suppress experimental autoimmune encephalomyelitis, rheumatoid arthritis, systemic lupus erythematosus, type I diabetes and inflammatory bowel disease [71]. Vitamin D hormone stimulates TGF-β1 and interleukin 4 (IL-4) production, which in turn suppresses inflammatory T cell activity [71]. Calcitriol attenuates the expression of experimental murine lupus [72]. In the kidney, 1,25(OH)2D3 also regulates mesangial cell smooth muscle phenotypes in a TGF-β1-mediated manner [73] and ameliorates glomerular injury in rats with experimental glomerulonephritis [74].
Difference between compounds
Based on the available data, it is difficult to draw any conclusion on the difference between individual forms of a vitamin D analogue. Two large cohort studies did attempt to perform head-to-head comparison between analogues. In an observational study of 7731 prevalent haemodialysis patients, Tentori et al. [20] found that mortality was significantly lower in patients treated with doxercalciferol and paricalcitol versus calcitriol in the unadjusted survival model. In adjusted models, however, this difference was not statistically significant. In another study of 67 399 prevalent haemodialysis patients, Teng et al. [21] found that the mortality rate was 16% lower (95% CI 10–21%) among paricalcitol-treated patients than calcitriol-treated ones. It could be argued that the latter study had a much larger sample size and longer follow-up as compared to the former one (36 months versus 9 months). Nonetheless, any observed difference between individual forms of a vitamin D analogue could equally be explained by the difference in the effective dosage. At this moment, it is impossible to recommend one product over another, although newer compounds with a lower risk of hypercalcaemia might be preferable if high-dose therapy is needed.
Further direction
Although the results seem promising, definitive proof of the clinical benefits by randomized control trial would be necessary before one could advocate the routine use of vitamin D analogues for the treatment of CKD patients. In fact, therapy with a vitamin D analogue is not without risk; hypercalcaemia or elevated calcium/phosphate product is always a concern. Around one-third of the ESRD patients treated with calcitriol for renal bone disease would have hypercalcaemia or elevated calcium/phosphate product [75,76]. It has been postulated that there exists some differential effect between calcitriol and other vitamin D analogues (for example, paricalcitol) on parathyroid hormone suppression and their relative incidence rate of hypercalcaemia and hyperphosphataemia [75–78]. Nonetheless, as many as 18% of dialysis patients treated with paricalcitol had persistent hypercalcaemia or elevated calcium/phosphate product [78]. In short, vitamin D therapy should not be taken lightly, and close monitoring of the biochemical profile is necessary.
Conclusions
While the only current indication for the use of activated vitamin D and its analogues in patients with CKD is the treatment of secondary hyperparathyroidism, a number of observational studies show that the use of activated vitamin D therapy in dialysis and pre-dialysis CKD patients is associated with a superior survival rate. In addition, animal studies and available, though limited, human data suggest that vitamin D therapy may have an anti-proteinuric effect and possibly reduce the rate of renal function deterioration. In addition to its primary role in calcium homeostasis and bone mineralization, vitamin D has a variety of additional functions including suppression of the renin–angiotensin system, direct cardiovascular, metabolic and immunomodulating effects. Putative mechanisms of action responsible for the lower mortality and progression of CKD associated with vitamin D therapy are summarized in Table 1. The wealth of experimental evidence and observational studies shed light on potential areas of further clinical trials that are necessary to test whether vitamin D therapy would confer clinically meaningful benefits to CKD patients.
Acknowledgments
This work was supported by the CUHK research accounts 6901031 and 8500282.
Conflict of interest statement. None declared.
References
- 1.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 2.Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy. A position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 3.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16:101–105. doi: 10.1046/j.1525-139x.2003.16025.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilmer WA, Rovin BH, Hebert CJ, et al. Management of glomerular proteinuria: a commentary. J Am Soc Nephrol. 2003;14:3217–3232. doi: 10.1097/01.asn.0000100145.27188.33. [DOI] [PubMed] [Google Scholar]
- 6.MacKinnon M, Shurraw S, Akbari A, et al. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis. 2006;48:8–20. doi: 10.1053/j.ajkd.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 7.Li PK, Leung CB, Chow KM, et al. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47:751–760. doi: 10.1053/j.ajkd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Perna A, Gherardi G, et al. Chronic proteinuric nephropathies: outcomes and response to treatment in a prospective cohort of 352 patients with different patterns of renal injury. Am J Kidney Dis. 2000;35:1155–1165. doi: 10.1016/s0272-6386(00)70054-0. [DOI] [PubMed] [Google Scholar]
- 9.da Cunha V, Tham DM, Martin-McNulty B, et al. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis. 2005;178:9–17. doi: 10.1016/j.atherosclerosis.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa T, Handa Y, Uematsu Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 12.Panda DK, Miao D, Tremblay ML, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dardenne O, Prud’homme J, Arabian A, et al. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 16.Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 17.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 18.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 20.Tentori F, Hunt WC, Rohrscheib MR, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 21.Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Ahmadzadeh S, Anderson JE, et al. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 23.Shoben AB, Rudser KD, de Boer IH, et al. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19:1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz U, Amann K, Orth SR, et al. Effect of 1,25(OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998;53:1696–1705. doi: 10.1046/j.1523-1755.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 25.Hirata M, Makibayashi K, Katsumata K, et al. 22-Oxacalcitriol prevents progressive glomerulosclerosis without adversely affecting calcium and phosphorus metabolism in subtotally nephrectomized rats. Nephrol Dial Transplant. 2002;17:2132–2137. doi: 10.1093/ndt/17.12.2132. [DOI] [PubMed] [Google Scholar]
- 26.Mizobuchi M, Morrissey J, Finch JL, et al. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol. 2007;18:1796–1806. doi: 10.1681/ASN.2006091028. [DOI] [PubMed] [Google Scholar]
- 27.Migliori M, Giovannini L, Panichi V, et al. Treatment with 1,25-dihydroxyvitamin D3 preserves glomerular slit diaphragm-associated protein expression in experimental glomerulonephritis. Int J Immunopathol Pharmacol. 2005;4:779–790. doi: 10.1177/039463200501800422. [DOI] [PubMed] [Google Scholar]
- 28.Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R, Acharya M, Tian J, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 30.Szeto CC, Chow KM, Kwan BC, et al. Oral calcitriol for the treatment of persistent proteinuria in immunoglobulin A nephropathy: an uncontrolled trial. Am J Kidney Dis. 2008;51:724–731. doi: 10.1053/j.ajkd.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 31.Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 32.Li YC. Vitamin D and diabetic nephropathy. Curr Diab Rep. 2008;8:464–469. doi: 10.1007/s11892-008-0080-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Sun L, Wang Y, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73:163–171. doi: 10.1038/sj.ki.5002572. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Zhang Y, Ning G, et al. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci USA. 2008;105:15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klaus G. Renoprotection with vitamin D: specific for diabetic nephropathy? Kidney Int. 2008;73:141–143. doi: 10.1038/sj.ki.5002693. [DOI] [PubMed] [Google Scholar]
- 36.Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008;73:1355–1363. doi: 10.1038/ki.2008.35. [DOI] [PubMed] [Google Scholar]
- 37.Andress DL. Vitamin D treatment in chronic kidney disease. Semin Dial. 2005;18:315–321. doi: 10.1111/j.1525-139X.2005.18408.x. [DOI] [PubMed] [Google Scholar]
- 38.Dusso AS, Brown AL. Mechanisms of vitamin D action and its regulation. Am J Kidney Dis. 1998;32(Suppl 2):S13–S24. doi: 10.1053/ajkd.1998.v32.pm9808140. [DOI] [PubMed] [Google Scholar]
- 39.Kristal-Boneh E, Froom P, Harari G, et al. Association of calcitriol and blood pressure in normotensive men. Hypertension. 1997;30:1289–1294. doi: 10.1161/01.hyp.30.5.1289. [DOI] [PubMed] [Google Scholar]
- 40.Lind L, Hänni A, Lithell H, et al. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995;8:894–901. doi: 10.1016/0895-7061(95)00154-H. [DOI] [PubMed] [Google Scholar]
- 41.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 42.Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens. 1990;3:903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 43.Imaoka M, Morimoto S, Kitano S, et al. Calcium metabolism in elderly hypertensive patients: possible participation of exaggerated sodium, calcium and phosphate excretion. Clin Exp Pharmacol Physiol. 1991;18:631–641. doi: 10.1111/j.1440-1681.1991.tb01637.x. [DOI] [PubMed] [Google Scholar]
- 44.Krause R, Buhring M, Hopfenmuller W, et al. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 45.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 46.Lind L, Wengle B, Wide L, et al. Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidiol) is dependent on plasma renin activity and calcium status. Am J Hypertens. 1989;2:20–25. doi: 10.1093/ajh/2.1.20. [DOI] [PubMed] [Google Scholar]
- 47.Pfeifer M, Begerow B, Minne HW, et al. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 48.Kimura Y, Kawamura M, Owada M, et al. Effectiveness of 1,25-dihydroxyvitamin D supplementation on blood pressure reduction in a pseudohypoparathyroidism patient with high renin activity. Intern Med. 1999;38:31–35. doi: 10.2169/internalmedicine.38.31. [DOI] [PubMed] [Google Scholar]
- 49.Park CW, Oh YS, Shin YS, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33:73–81. doi: 10.1016/s0272-6386(99)70260-x. [DOI] [PubMed] [Google Scholar]
- 50.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int. 2005;68:1973–1981. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhou C, Lu F, Cao K, et al. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 53.Nakane M, Ma J, Ruan X, et al. Mechanistic analysis of VDR-mediated renin suppression. Nephron Physiol. 2007;107:35–44. doi: 10.1159/000106792. [DOI] [PubMed] [Google Scholar]
- 54.Pörsti IH. Expanding targets of vitamin D receptor activation: downregulation of several RAS components in the kidney. Kidney Int. 2008;74:1371–1373. doi: 10.1038/ki.2008.424. [DOI] [PubMed] [Google Scholar]
- 55.Freundlich M, Quiroz Y, Zhang Z, et al. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008;74:1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- 56.Kim HW, Park CW, Shin YS, et al. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102:c21–c29. doi: 10.1159/000088295. [DOI] [PubMed] [Google Scholar]
- 57.Walters MR, Ilenchuk TT, Claycomb WC. 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+uptake by cultured adult rat ventricular cardiac muscle cells. J Biol Chem. 1987;262:2536–2541. [PubMed] [Google Scholar]
- 58.O’Connell TD, Berry JE, Jarvis AK, et al. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272:H1751–H1758. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz K, Lecarpentier Y, Martin JL, et al. Myosin isoenzymic distribution correlates with speed of myocardial contraction. J Mol Cell Cardiol. 1981;13:1071–1075. doi: 10.1016/0022-2828(81)90297-2. [DOI] [PubMed] [Google Scholar]
- 60.Pagani ED, Julian FJ. Rabbit papillary muscle myosin isozymes and the velocity of muscle shortening. Circ Res. 1984;54:586–594. doi: 10.1161/01.res.54.5.586. [DOI] [PubMed] [Google Scholar]
- 61.Andress D. Nonclassical aspects of differential vitamin D receptor activation: implications for survival in patients with chronic kidney disease. Drugs. 2007;67:1999–2012. doi: 10.2165/00003495-200767140-00003. [DOI] [PubMed] [Google Scholar]
- 62.Carthy EP, Yamashita W, Hsu A, et al. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 63.Panichi V, De PS, Andreini B, et al. Calcitriol modulates in vivo and in vitro cytokine production: a role for intracellular calcium. Kidney Int. 1998;54:1463–1469. doi: 10.1046/j.1523-1755.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 64.Staeva-Vieira TP, Freedman LP. 1,25-Dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168:1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 65.Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem. 2004;279:35798–35802. doi: 10.1074/jbc.M404865200. [DOI] [PubMed] [Google Scholar]
- 66.Tukaj C, Trzonkowski P, Kubasik-Juraniec J, et al. Quantifying division of aortal smooth muscle cells in culture stimulated by 1,25(OH)2D3. J Steroid Biochem Mol Biol. 2007;103:525–528. doi: 10.1016/j.jsbmb.2006.12.100. [DOI] [PubMed] [Google Scholar]
- 67.Chiu KC, Chu A, Go VLW, et al. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 68.Wehmeier KR, Mazza A, Hachem S, et al. Differential regulation of apolipoprotein A-I gene expression by vitamin D receptor modulators. Biochim Biophys Acta. 2008;1780:264–273. doi: 10.1016/j.bbagen.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 69.Wehmeier K, Beers A, Haas MJ, et al. Inhibition of apolipoprotein AI gene expression by 1, 25-dihydroxyvitamin D3. Biochim Biophys Acta. 2005;1737:16–26. doi: 10.1016/j.bbalip.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Abe J, Takita Y, Nakano T, et al. A synthetic analogue of vitamin D3, 22-oxa–1α,25-dihydroxyvitamin D3, is a potent modulator of in vivo immunoregulating activity without inducing hypercalcemia in mice. Endocrinology. 1989;124:2645–2647. doi: 10.1210/endo-124-5-2645. [DOI] [PubMed] [Google Scholar]
- 71.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 72.Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/1 mice. Autoimmunity. 1992;12:143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 73.Abe H, Iehara N, Utsunomiya K, et al. A vitamin D analog regulates mesangial cell smooth muscle phenotypes in a transforming growth factor-β type II receptor-mediated manner. J Biol Chem. 1999;274:20874–20878. doi: 10.1074/jbc.274.30.20874. [DOI] [PubMed] [Google Scholar]
- 74.Makibayashi K, Tatematsu M, Hirata M, et al. A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol. 2001;158:1733–1741. doi: 10.1016/S0002-9440(10)64129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin KJ, Gonzalez EA. Vitamin D analogues for the management of secondary hyperparathyroidism. Am J Kidney Dis. 2001;38(Suppl 5):S34–S40. doi: 10.1053/ajkd.2001.28109. [DOI] [PubMed] [Google Scholar]
- 76.Martin KJ, Gonzalez EA, Gellens ME, et al. Therapy of secondary hyperparathyroidism with 19-nor-1alpha, 25-dihydroxyvitamin D2. Am J Kidney Dis. 1998;32:S61–S66. doi: 10.1053/ajkd.1998.v32.pm9808145. [DOI] [PubMed] [Google Scholar]
- 77.Wolf M, Thadhani R. Vitamin D in patients with renal failure: a summary of observational mortality studies and steps moving forward. J Steroid Biochem Mol Biol. 2007;103:487–490. doi: 10.1016/j.jsbmb.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]