Abstract
Cardiovascular morbidity and mortality are common in peritoneal dialysis patients. Metabolic syndrome (MES) is a medical condition with a clustering of major risk factors for cardiovascular diseases. In this review article, the various diagnostic criteria used in MES are discussed. It is proposed to use a modified National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III) criteria for the diagnosis of MES in peritoneal dialysis (PD) patients taking into consideration the scientific evidence and practicality. When three or more of the following criteria are satisfied in PD patients, obesity, high triglyceride, low high-density lipoprotein cholesterol (HDL-C), hypertension or dysglycaemia, they are diagnosed as having MES. Body mass index (BMI) with reference to ethnicity is suggested to replace waist circumference for diagnosing obesity. Epidemiology and outcome of PD patients with MES are highlighted. The adverse sequelae of obesity appear to be primarily due to fat mass rather than non-fat mass, possibly related to the pro-inflammatory effect of adipose tissue. Whilst there are therapies to tackle MES in PD patients, more conclusive data in human studies to see clinically improved outcomes with such strategies are needed.
Keywords: metabolic syndrome, obesity, peritoneal dialysis
Introduction
Metabolic syndrome (MES) is a medical condition with a clustering of major risk factors for cardiovascular diseases and type 2 diabetes [1]. It was also previously termed syndrome X [2], or syndrome of insulin resistance [3]. There are several definitions of MES worldwide. In 1998, Alberti and Zimmet proposed, for the first time, a definition for MES for World Health Organization (WHO) [4]. It consists of insulin resistance and/or dysglycaemia, plus two or more of the following conditions: (i) hypertension = known hypertensive or blood pressure (BP) ≥160/90 mmHg (modified to ≥140/90 mmHg in 1999 [5]); (ii) dyslipidaemia = triglyceride (TG) ≥1.7 mmol/L and/or high-density lipoprotein cholesterol (HDL-C) <0.9 mmol/L in men or <1.0 mmol/L in women; (iii) obesity = waist–hip ratio (WHR) >0.9 in men or >0.85 in women and/or body mass index (BMI) >30 kg/m2 and (iv) microalbuminuria = urinary albumin excretion rate ≥20 μg/min or albumin:creatinine ratio ≥20 mg/g.
Dysglycaemia includes known diabetes or impaired glucose tolerance (IGT) or fasting plasma glucose (PG) ≥6.1 mmol/L. There is still no international consensus on a ‘normal range’ for insulin resistance. With euglycaemic clamp technique, insulin resistance is defined in this WHO 1998 criterion as glucose uptake below lowest quartile for background population under investigation [4].
In 1999, the European Group for the Study of Insulin Resistance (EGIR) also proposed a similar definition [6], which requires the measurement of insulin resistance. This EGIR criterion was most impressive by introducing the waist circumference to define obesity, instead of using BMI and WHR. In 2001, the National Cholesterol Education Program (NCEP) Expert Panel (Adult Treatment Panel III) proposed a more simple diagnostic criterion for clinical identification of MES in their third report [7]. According to the NCEP criterion, having three or more of the following conditions are considered diagnostic for MES: obesity (waist circumference >102 cm in men or >88 cm in women), high BP (BP ≥130/85 mmHg or on treatment), dysglycaemia (known diabetes or fasting PG ≥6.1 mmol/L, modified to ≥5.6 mmol/L in 2005 [8]), elevated fasting plasma TG (≥1.7 mmol/L), low HDL-C concentration (<1.0 mmol/L in men or <1.3 mmol/L in women). Due to its simplicity and clinical relevance, the NCEP definition for MES is nowadays the one being most widely used in general populations.
Other definitions of MES include American Association of Clinical Endocrinologists (AACE) criteria proposed in 2003 [9] and International Diabetes Federation (IDF) criteria in 2005 [1]. In particular, the most recently proposed IDF criterion modified the NCEP criterion and highlighted the essential element of central obesity and the need to consider ethnicity in its definition. Table 1 summarizes these definitions and variations in the thresholds for each category in the different criteria.
Table 1.
Comparison of the various definitions of metabolic syndrome in general populations
| WHO, 1998 [4] | EGIR, 1999 [6] | NCEP ATPIII, 2001 [7] | IDF, 2005 [1] | |
|---|---|---|---|---|
| Insulin resistance | Known dysglycaemia (diabetes/IFG/IGT) and/or insulin resistance | Insulin resistance | – | – |
| Plus ≥2 of the following: | Plus ≥2 of the following: | ≥3 of the following: | ||
| Obesity | BMI >30 kg/m2 or waist-to-hip ratio for men: >0.9; for women: >0.85 | Waist circumference for men: ≥94 cm; for women: ≥80 cm | Waist circumference for men: >102 cm; for women: >88 cm | Waist circumference for men: ≥94 cm; for women: ≥80 cm (a prerequisite) |
| Plus ≥2 of the following: | ||||
| Dyslipidaemia | TG: ≥1.7 mmol/L; or HDL-C for men: <0.9 mmol/L; for women: <1.0 mmol/L | TG: >2.0 mmol/L; or HDL-C: <1.0 mmol/L | TG: ≥1.7 mmol/L | TG: ≥1.7 mmol/L |
| Low HDL-C: <1.0 mmol/L in men; or <1.3 mmol/L in women | Low HDL-C: <1.0 mmol/L in men; or <1.3 mmol/L in women | |||
| Hypertension | On treatment or BP: ≥160/ 90 mmHga | On treatment or BP ≥140/ 90 mmHg | On treatment or BP ≥130/ 85 mmHg | On treatment or BP ≥130/ 85 mmHg |
| Microalbuminuria | Urinary albumin excretion ≥20 μg/min or albumin:creatinine ratio ≥20 mg/g | – | – | – |
| Dysglycaemia | Fasting PG ≥6.1 mmol/L | Fastingb PG ≥6.1 mmol/L | Fasting PG ≥5.6 mmol/L |
WHO, World Health Organization; EGIR, European Group for the study of Insulin Resistance; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; AACE, American Association of Clinical Endocrinology; IDF, International Diabetes Federation; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; BMI, body mass index; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; BP, blood pressure; PG, plasma glucose; OGTT, oral glucose tolerance test.
aBP cutoff value of the WHO 1998 criteria was revised to 140/90 mmHg in 1999 [5].
bFasting PG cutoff level of the NCEP ATPIII 2001 criteria was modified to 5.6 mmol/L in 2005 [8].
Pathophysiology of metabolic syndrome
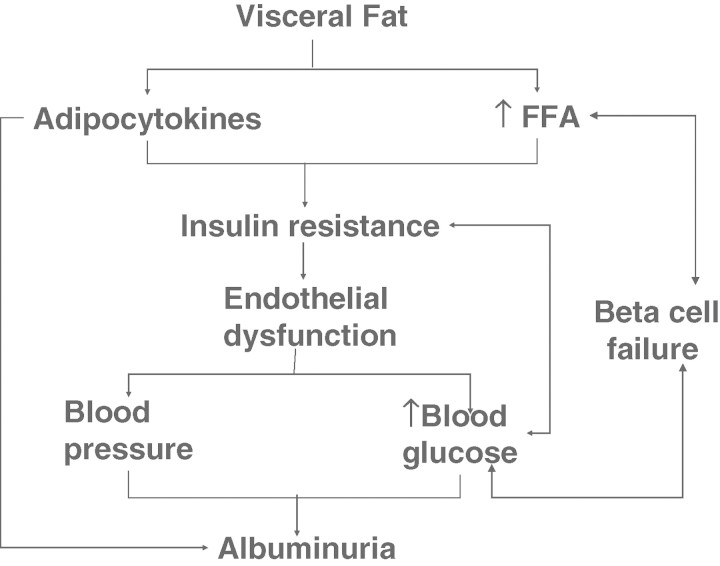
The key element of the constellation of features in MES is obesity as highlighted by the revised criteria for MES by IDF [1,10]. Obesity, especially central obesity, is associated with increased visceral adipose tissue that, being metabolically very active, releases a substantial amount of free fatty acids (FFA) and hence associated with a high serum FFA level.
Adipose tissue can be considered as the largest secretory organ in the body [11,12]. Adipocytes produce a wide range of signalling protein and factors termed adipocytokines [13]. Some of the major adipocytokines include tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), plasminogen activator inhibitor (PAI-1), leptin, monocyte chemotactic protein-1, macrophage migration inhibitory factor and adiponectin. These serve as the signals for the effects of adipocytes on insulin resistance, inflammation, dyslipidaemia, hypertension, endothelial dysfunction and atherosclerosis [14–24]. Excessive fatty acids are also associated with insulin resistance, proinflammatory state and pro-thrombotic state [25,26]. Long-standing hypertension and abnormal blood glucose levels can lead to damage of the kidneys, resulting in albuminuria and even renal failure (see Figure 1).
Fig. 1.
Association between visceral fat and other elements of metabolic syndrome (FFA: free fatty acid).
Factors to be considered on the diagnostic criteria
Obesity—ethnic issues
Conventionally, obesity is defined as body fat >25% in males and >35% in females for young adults [27]. In young Caucasians, these body fat percentages correspond to a BMI of 30 kg/m2 while a BMI of 25 kg/m2 corresponds to body fat percentages of 20 in males and 30 in females [28]. In accord to these, a BMI cutoff point of ≥25–29.9 kg/m2 is defined for overweight while ≥30 kg/m2 for obesity [29].
In the early 1990s, epidemiological studies in Asians have shown that the threshold value of anthropometric indexes for association with cardiovascular risk factors was considerably lower than those conventionally used in Caucasians [30,31]. This has led to the proposed Asian definition of obesity: BMI ≥ 25 kg/m2; waist circumference ≥ 80 cm in women and ≥90 cm in men [32]. This was followed by a prolific number of reports, albeit many were cohort-based and cross-sectional in nature, on different definitions of obesity in different populations [33,34]. Nevertheless, there is now strong evidence showing that Asian people have more body fat than their Caucasian counterparts for the same BMI [35,36] suggesting the ‘international’ cutoff for general obesity at BMI ≥30 kg/m2 is too high for Asians. Similar arguments are also applicable to central obesity with the waist circumference cutoff levels among Caucasians at 102 cm (men) and 88 cm (women) are too high and should be modified to 90 cm and 80 cm (men and women respectively) among Asians [37]. The IDF criteria for MES has taken these evidences into consideration and emphasized the ethnic issue in its cutoff levels for obesity [1].
Waist circumference versus BMI
In the first MES criterion by WHO, BMI and WHR were used to define obesity. Nowadays, waist circumference is considered to be a more important surrogate marker than BMI or WHR for the harmful effects of obesity. This has been supported by the findings that visceral fat measurement on CT scan or magnetic resonance imaging correlate better with waist circumference than WHR [38–40]. However, among renal patients undergoing peritoneal dialysis, waist circumference may not reliably reflect abdominal visceral fat content. This is due to the presence of Tenckhoff catheter in situ, lax skin condition after repeated distention of the abdomen by peritoneal dialysis fluid and potential residual peritoneal dialysate inside the abdomen cavity. With all these considerations, BMI may remain the best anthropometric parameter to measure obesity in renal patients undergoing peritoneal dialysis. It has to be emphasized that BMI remains a good measure in defining obesity. One main reason is the widely reported validity of using BMI in categorizing body composition and body fat content [41,42]. There is no doubt that central adiposity does more harm than general adiposity. However, with the above major confounders in measuring the waist properly in patients on peritoneal dialysis, BMI should be the better alternative.
Plasma glucose levels in PD patients
Genuine fasting state could not be achieved in most peritoneal dialysis (PD) patients because of the continuous glucose absorption from dialysate. There is little doubt that PD patients with fasting glucose over 200 mg/dL (11.1 mmol/L) are definitely diabetic, but those with fasting glucose between 126 and 200 mg/dL (7.0 to 11.1 mmol/L) may be considered as having IGT and are not truly diabetic [43]. In addition to the harmful metabolic effect of glucose load on diabetic patients treated by conventional glucose-based peritoneal dialysis solution, newer evidence has accrued that the adverse effect of glucose load applies to peritoneal dialysis patients without pre-existing diabetes mellitus. We studied 252 non-diabetic Chinese patients newly started on continuous ambulatory peritoneal dialysis for the fasting plasma glucose level 1 month after peritoneal dialysis [44]. Fasting plasma glucose was measured by means of a conventional method after an overnight fast, but with continuation of PD therapy with 1.5% dextrose dialysate. We found that the fasting plasma glucose level correlated significantly with the baseline serum C-reactive protein level. Most importantly, our results showed that even mild hyperglycaemia after peritoneal dialysis, with the fasting plasma glucose level >5.6 mmol/L (100 mg/dL), is associated with worse survival. By multivariate analysis with the Cox proportional hazard model, every 0.6 mmol/L (10 mg/dL) increase in the fasting plasma glucose level conferred 1.6% excess hazard of all-cause mortality [44]. Although a high fasting plasma glucose level 1 month after starting peritoneal dialysis is probably a surrogate indicator of elderly subjects with multiple comorbid conditions and systemic inflammation, fasting plasma glucose level remained an independent predictor of survival after adjusting for age, C-reactive protein level and Charlson's comorbidity score in our study, suggesting a direct detrimental effect of high glucose level [44].
Proposed diagnostic criteria for metabolic syndrome in PD patients
With all these considerations for diagnosis of metabolic syndrome in PD patients, we propose the following criteria. Modified from NCEP ATP III, 2001 criteria, whenever there are three or more of the following five factors: obesity, high triglyceride, low HDL-C, hypertension or dysglycaemia in PD patients, they are considered having metabolic syndrome (Table 2). These are based on both scientific evidence and practical use of the criteria for making the diagnosis. NCEP criteria, instead of IDF, are chosen since there is accumulating evidence that suggests that the former is much better associated with clinical outcomes such as incident coronary heart disease [45,46]. In addition, subjects identified by NCEP criteria are metabolically less favourable and have more insulin resistance than those identified by IDF criteria [47]. Compared to subjects identified by the NCEP definition, subjects identified in excess by IDF showed lower IMT and plaque extent indistinguishable from MES-free subjects [47].
Table 2.
Proposed diagnostic criteria for metabolic syndrome in peritoneal dialysis (PD) patients
| Modified from NCEP ATP III, 2001 criteria | |
|---|---|
| ≥3 of the following: | |
| Obesity | 1. BMIa >30 kg/m2 for Caucasians |
| BMIa >25 kg/m2 for Asians | |
| Dyslipidaemia | 2. TG: ≥1.7 mmol/L |
| 3. Low HDL-C: <1.0 mmol/L in men; or <1.3 mmol/L | |
| in women | |
| Hypertension | 4. BP ≥130/85 mmHg or hypertension on treatment |
| Dysglycaemia | 5. Fastingb PG ≥5.6 mmol/L or diabetic on treatment |
aBody weight for BMI in PD patients is measured either with a dry abdomen or with PD dialysate in abdomen minus X kg (X is the volume in litres of PD dialysate infused).
bFasting plasma glucose (PG) in PD patients is measured by means of a conventional method after an overnight fast, but with continuation of PD therapy with 1.5% dextrose dialysate.
NCEP ATP III, National Cholesterol Education Programme Adult Treatment Panel III; BMI, body mass index; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; BP, blood pressure.
Following the above discussions, we propose to use BMI instead of waist circumference for our PD patients to define obesity. Body weight for BMI in PD patients is measured either with a dry abdomen or with PD dialysate in the abdomen minus X kg (X is the volume in litres of PD dialysate infused). The body weight should be measured when the patient is not fluid overloaded or dehydrated. Fasting plasma glucose (PG) in PD patients is measured by means of a conventional method after an overnight fast, but with continuation of PD therapy with 1.5% dextrose dialysate. This is practically more convenient for our patients coming back for follow-up without the need to drain dry the abdomen the night before. The fasting PG cutoff level of the NCEP ATPIII 2001 criterion was already modified to 5.6 mmol/L in 2005 [8]. In addition, our previous study showed that the fasting plasma glucose level (based on oral fast but without ‘peritoneal fast’) >5.6 mmol/L (100 mg/dL) was already associated with worse survival [44]. Therefore, we used the fasting plasma glucose level (oral fast without ‘peritoneal fast’) >5.6 mmol/L (100 mg/dL) as the criteria for dysglycaemia.
Epidemiology in the general population, CKD and PD patients
Using the NCEP criterion, the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994) [48] reported the age-adjusted prevalence of MES among the US adult general population as 23.7%, affecting 47 million people. Using the same criterion, ∼15–25% of the world's general population has features of MES [25]. In a population-based study conducted in Hong Kong using the same criterion, 16.7% (age- and gender-adjusted) of the 2893 subjects had MES [49]. The prevalence of MES is much higher in an at-risk group as compared to the general population. The corresponding figures in patients with type 2 diabetes in Northern Europe and Hong Kong were reported to be 80% and 60%, respectively [50,51].
It has previously been reported that those with MES have increased risk of developing chronic kidney disease (CKD). Weir et al. [52] showed that those with three or more criteria of MES had an adjusted odds ratio (OR) of 2.21 of developing CKD. In accord to this, Tanaka's study in Japan involving >2000 subjects also showed that each of the risk factors in MES (abdominal obesity, high TG, low HDL-C, high fasting PG and hypertension) was associated with the increased prevalence of chronic kidney disease [53]. At the same time, Tanaka et al. [53] found that the prevalence of chronic kidney disease increased with the number of MES risk factors such that the prevalence of chronic kidney disease was 11.0% in those with no metabolic risk factors, 12.5% with one, 15.4% with two, 20.7% with three, 24.2% with four and 20.0% with five, respectively. Using the absence of MES risk factors as reference, the adjusted OR (95% CI) of developing chronic kidney disease when the number of MES risk factors increased from 1 to 5 were 1.029 (P-value: NS), 1.062 (P-value: NS), 1.206 (P-value: NS), 1.744 (P = 0.0002) and 2.109 (P = 0.0077), respectively [53]. Tanaka's study used the modified NCEP criteria for MES.
A similar study in a Chinese population showed that the prevalence of chronic kidney disease (estimated-GFR <60 mL/min) and elevated serum creatinine ≥100.8 μmol/L (1.14 mg/dL) in men and ≥85.7 μmol/L (0.97 mg/dL) in women increases with the number of components of metabolic syndrome [54]. In those without any of the components of MES, there was a prevalence of 1.4% chronic kidney disease and this increased to 6.7% if the patients had five components of MES. Likewise, the prevalence of elevated serum creatinine was 4.6% in patients with no components of MES and this increased to 9.5% in those with five components of MES as defined by the NCEP criteria [54].
In a study done by Johnson's group, the prevalence of MES was 30.5% in a group of 200 subjects with chronic kidney disease stages 4 and 5 [55]. This group included 49 peritoneal dialysis, 78 haemodialysis and 73 pre-dialysis subjects. MES was independently predicted by older age, peritoneal dialysis and Maori/Pacific Islander origin. In this study, the prevalence of MES was highest among those on peritoneal dialysis (50%) [55]. Another recent retrospective, cross-sectional study of 202 incident dialysis patients (94% haemodialysis and 6% peritoneal dialysis) examined the prevalence of the metabolic syndrome at the time of renal replacement therapy initiation [56]. Females represented 39.1% and blacks composed 34.7% of the study population, respectively. Diabetes was the aetiology of ESRD in 44.6% of the patients. Using NCEP criteria, the overall prevalence of the metabolic syndrome was 69.3% in that population and was especially prevalent among diabetic, female and white ESRD patients. Using the proposed modified NCEP criteria in Table 2, our cohort of 212 CAPD patients showed a prevalence of 53.3% (113/212) having MES (PWH unpublished data).
Outcome
MES is associated with a raised level of pro-inflammatory cytokines such as IL-6, TNF-α and a reduced level of nitric oxide and adiponectin. This leads to increased inflammation, vasoconstriction and thrombosis, and hence, an accelerated process of atherosclerosis formation [15,17,18,22,24,57].
The dialysis population has a much higher risk of cardiovascular mortality compared with the general population [58,59]. It has also been shown that among subjects with chronic kidney disease stages 4 and 5, those with MES have a significantly higher risk of mortality (P < 0.05) compared to those without risk factors of MES over a 24-month follow-up [55]. At the same time, in United States, subjects with end-stage renal failure tend to have a higher BMI compared with the general population [60].
A raised BMI in the general population is associated with higher risk of mortality [61–66]. However, it is still not clear whether obesity leads to a better or worse survival outcome in patients on dialysis. Quite a number of studies have shown that in both haemodialysis and peritoneal dialysis patients, higher BMI is associated with improved survival [67–70]. At the same time, some studies showed that survival advantage associated with obesity among chronic dialysis patients is significantly less for peritoneal dialysis patients as compared to haemodialysis patients [71,72]. On the other hand, some studies have shown that a higher degree of obesity leads to worse survival in peritoneal dialysis patients [73]. This negative impact of obesity is associated with a higher hazard ratio of developing peritonitis [74], peritoneal dialysis technique failure [73] and more rapid loss of residual renal function [75].
One reason for the varying association between BMI and survival could be the effect of body composition [76]. In a study on peritoneal dialysis patients, those with a high BMI due to increased fat mass have a worse survival compared to those with a high BMI due to non-fat mass [76]. In other words, incident PD patients with high BMI and normal or high muscle mass have the best survival, and Ramkumar N et al. [76] suggested that PD patients should be encouraged to gain muscle mass rather than fat mass. Our previous study also showed that every 1% increase in lean body mass is associated with a 10% reduction in mortality in our PD patients [69]. In peritoneal dialysis patients, it has been shown that BMI is correlated with IL-6 (r = 0.43, P = 0.004), TNF-α (r = 0.36, P = 0.018), leptin (r = 0.68, P < 0.001) and C-reactive protein (CRP) (r = 0.31, P = 0.044) [77]. Thus, obesity in patients on peritoneal dialysis may be considered as a pro-inflammatory state [77].
Patients on peritoneal dialysis, compared with those on haemodialysis, might be more at risk of glucose dysregulation, and thus MES. Peritoneal dialysis solutions contain a sizable amount of glucose. Sixty to eighty percent of the glucose instilled into the peritoneal cavity is absorbed, corresponding to 100–300 g of glucose per day [78]. Our group has shown that increased subcutaneous insulin is required in diabetic patients recently commenced on peritoneal dialysis [79]. We also noted a high rate of developing new onset hyperglycaemia among non-diabetic subjects started on peritoneal dialysis [44]. Four weeks after initiation of peritoneal dialysis in 252 non-diabetic Chinese patients, we found that 59 (23.4%) of them developed new onset hyperglycaemia (fasting PG ≥7.0 mmol/L or 125 mg/dL) [48 patients (19.0%) had fasting PG between 7.0 and 11.1 mmol/L (126–200 mg/dL) and 11 patients (4.4%) had fasting PG >11.1 mmol/l (200 mg/dL)] [44]. De novo diabetes mellitus in non-diabetic patients on peritoneal dialysis has also been noted in previous studies and can be in the range of 5% [80,81].
Interventions
We should be alert to the possibility of developing MES among our patients started on dialysis. Interventions for MES mainly consist of lifestyle modification. Specific treatment for each of the components is also indicated in selected patients to optimize the effects.
Abnormal glucose
Glycaemic control in PD patients consists of lifestyle changes, hypoglycaemic agents and non-glucose-based dialysis solutions. Lifestyle changes take effect through diet, exercise and body weight control. The US Diabetes Prevention Program has demonstrated that the incidence of the metabolic syndrome in a volunteer non-uraemic population was reduced in the lifestyle group compared with placebo [82]. Three-year cumulative incidences of MES were 51% and 34% in the placebo and lifestyle groups, respectively [82].
Thiazolidinediones may also aid the diabetic control. Apart from reducing insulin resistance, thiazolidinediones (peroxisome proliferator-activated receptor-γ agonists) also have a specific anti-inflammatory effect that may be beneficial in patients with renal failure [83]. We previously performed a randomized study in which 52 patients with type 2 diabetes on peritoneal dialysis therapy administered a constant dosage of subcutaneous insulin with stable glycaemic control were randomly assigned to the use of either a fixed dose of rosiglitazone (RSG) plus insulin or insulin alone [84]. After 24 weeks, the percentage reduction in insulin dosage was significantly greater in the RSG group than the control group (−21% versus −0.5%, P = 0.02). Moreover, there appeared to be an anti-inflammatory effect with RSG. At baseline, the two groups had statistically similar CRP levels (RSG versus insulin: 9.35 versus 8.63 mg/L, P = 0.836) while at the end of 24 weeks, the RSG groups had significantly lower CRP levels than the control group (2.21 versus 8.59 mg/L, P = 0.03). Recent studies have shown that more biocompatible and non-glucose-containing dialysis fluids, e.g. icodextrin and amino acid solutions, seem to be associated with improvements in glycaemic control in the diabetic patients on peritoneal dialysis [85].
Hypertension
Most subjects with chronic kidney disease and especially those on dialysis tend to have hypertension that usually requires treatment with anti-hypertensive agents. Their BP is partly related to their fluid status, and adequate fluid removal during dialysis can aid in reduction of hypertension.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (The JNC-7 Report) defined BP in the range 120–139/80–89 mmHg as ‘Prehypertension’ [86]. This suggestion is in accord to the concept of ‘prediabetes’ with fasting PG in the range 5.6–6.9 mmol/L as impaired fasting glucose while frank diabetes is defined as the fasting PG level ≥7.0 mmol/L. The JNC-7 report also recommends initiating drug therapy for subjects with ‘Prehypertension’ with compelling indications such as chronic kidney disease, heart failure and diabetes. For patients with chronic kidney disease or diabetes, BP <130/80 mmHg is targeted as treatment goal [86]. Of note is our recent study using an ACE inhibitor in patients on PD [87]. There was benefit of ACE inhibitor in the preservation of residual renal function in addition to the BP control in PD patients [87].
Obesity
One of the important factors for the constellation of features in MES is obesity. In the general population, reducing obesity, especially reducing visceral obesity, will improve the lipid profile, improve insulin sensitivity and thus better insulin and glucose profile. These will subsequently reduce the susceptibility to thrombosis, decrease the levels of inflammatory markers and improve endothelial function. All these serve to alleviate the risk of coronary heart disease [88]. It was previously shown that plausible relations exist between inflammatory biomarkers, such as IL-6 and high-sensitivity C-reactive protein and regional fat distribution in ESRD patients [89] Also, increased adipose tissue signalling was found in patients with uraemia that might share some characteristics with the metabolic syndrome of obesity [90].
In PD patients, general measures to reduce weight are similar to that in the non-dialysis population. A multidisciplinary approach to weight reduction is more likely to be successful [91]. This includes individualized meal plans according to energy and nutritional requirements and increasing physical activity. Surgical means such as laparoscopic banding should only be considered for extreme obesity. In the general population, a recent meta-analysis suggested that drugs such as orlistat, sibutramine and rimonabant can modestly reduce weight, have differing effects on cardiovascular risk profiles and have specific adverse effects [92]. However, pharmacotherapy for PD patients with oral anti-obesity drugs is currently not an option due to their limited safety profiles among patients on dialysis.
In the peritoneal dialysis population, avoiding or minimizing peritoneal dialysate glucose, e.g. using icodextrin and amino acid solutions, may aid weight control. A trial using icodextrin compared with 2.5% dextrose peritoneal dialysis fluid showed that patients receiving icodextrin had no increase in weight after 52 weeks, in contrast to a weight gain of almost 2 kg in the dextrose group [93]. Adjusted body weight during the 52-week study for the icodextrin arm was also significantly lower when compared to baseline [93].
Recommendations for weight reduction, especially fat mass, in peritoneal dialysis patients with MES have theoretical advantages. Unfortunately, so far, there is little evidence that weight reduction is associated with improved patient outcomes in peritoneal dialysis.
Hyperlipidaemia
Dyslipidaemia can be treated with reduction in the amount of dietary cholesterol or fats, use of lipid-modifying drugs such as statins or fibrates. There are few large studies concentrating on use of these drugs in the peritoneal dialysis population. Wanner et al. conducted the Deutsche Diabetes Dialyse Studie (4D study) [94] which recruited subjects with type 2 diabetes mellitus receiving maintenance haemodialysis. The 1255 subjects were randomly assigned to receive atorvastatin or placebo. At 4 weeks, the median low-density lipoprotein cholesterol (LDL-C) level had a reduction of 42% in the atorvastatin group, from 3.13 mmol/L (121 mg/dL) to 1.86 mmol/L (72 mg/dL), but only 1.3% in the placebo group, from 3.23 mmol/L (125 mg/dL) to 3.10 mmol/L (120 mg/dL). However, after a median follow-up of 4 years, atorvastatin had no statistically significant effect on the composite primary endpoint of cardiovascular death, nonfatal myocardial infarction and stroke in patients with diabetes receiving haemodialysis. So far we do not have large-scale lipid-lowering trials on survival in PD patients.
The LANDMARK (Longitudinal Assessment of Numerous Discrete Modifications of Atherosclerotic Risk factors in Kidney disease) study [95] assessed a mixed group of haemodialysis, peritoneal dialysis and pre-dialysis patients. Despite having significant improvement in serum LDL-C (−30.9 mg/dL versus −12.7 mg/dL, P = 0.001), homocysteine (−6.95 versus −0.67 micromol/L, P < 0.001), systolic BP (−6.9 versus −0.2 mmHg, P = 0.049) and diastolic BP (−4.8 versus −1.0 mmHg, P = 0.043), there was no significant change noted in the carotid intima-media thickness or brachial artery reactivity, as outcome measures of atheroma burden and endothelial function [95]. It was concluded that multiple risk factor intervention programme was not associated with improvement in vascular structure or function in stage 4 or 5 patients with chronic kidney disease. Another study in chronic kidney disease subjects with MES randomized to intensive risk factor modification also did not show any significant difference compared to those on usual care (log-rank score 0.37, P = 0.54) [55].
Conclusions
In the general population, MES is associated with increased cardiovascular morbidity and mortality. The prevalence of MES is increasing worldwide, affecting ∼15–25% of the general population in most parts of the world. In peritoneal dialysis patients, the prevalence is in the range of >50%. Previous studies have shown that the presence of MES in those with end-stage renal failure predicts poor survival. Despite some reports that in dialysis patients, a high BMI is associated with a better survival, those with low muscle mass (and thus high fat mass) probably still have a worse survival compared to those with a high muscle mass.
There is evidence that increased cardiovascular risk in the peritoneal dialysis population is due to the interplay between traditional cardiovascular risk factors (metabolic factors) and inflammation. In the general population, improving the metabolic profile can significantly improve the cardiovascular risk. However, evidence in the dialysis population that interventions targeting individual elements of MES can improve outcomes is still pending. Thus, a large-scale interventional research study on the clinical outcome data in this area should be performed.
Acknowledgments
This study was supported in part by the Chinese University of Hong Kong (CUHK) Research Grant Account 6900570 and the Richard Yu CUHK PD Research Fund.
Conflict of interest statement. None declared.
References
- 1.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus WHO/NCD/NCS/99.2. Geneva, 1999.
- 6.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 9.Bloomgarden ZT. American Association of Clinical Endocrinologists (AACE) consensus conference on the insulin resistance syndrome: 25–26 August 2002 Washington, DC; Diabetes Care; 2003. pp. 1297–1303. [DOI] [PubMed] [Google Scholar]
- 10.Zimmet P, Magliano D, Matsuzawa Y, et al. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 11.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(Suppl 5):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 12.Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci. 2005;330:280–289. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 14.Bastard JP, Jardel C, Delattre J, et al. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2221–2222. [PubMed] [Google Scholar]
- 15.Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96:3042–3047. doi: 10.1161/01.cir.96.9.3042. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Peraldi P, Budavari A, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 18.Quehenberger P, Exner M, Sunder-Plassmann R, et al. Leptin induces endothelin-1 in endothelial cells in vitro. Circ Res. 2002;90:711–718. doi: 10.1161/01.res.0000014226.74709.90. [DOI] [PubMed] [Google Scholar]
- 19.Stephens JM, Pekala PH. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem. 1991;266:21839–21845. [PubMed] [Google Scholar]
- 20.Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 21.von Eynatten M, Schneider JG, Humpert PM, et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia: an association independent of systemic inflammation and insulin resistance. Diabetes Care. 2004;27:2925–2929. doi: 10.2337/diacare.27.12.2925. [DOI] [PubMed] [Google Scholar]
- 22.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead JP, Richards AA, Hickman IJ, et al. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 24.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 25.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 26.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6:177–181. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- 27.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization, Physical Status. The use and interpretation of anthropometry. Technical Report Series 854, Geneva, 1995. [PubMed]
- 29.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Expert Consultation on Obesity, Geneva, 3–5 June 1997. WHO/NUT/NCT/98.1. Technical Report Series Number 894, Geneva, 2000. [PubMed]
- 30.Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev. 2002;3:209–215. doi: 10.1046/j.1467-789x.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 31.Ko GT, Chan JC, Cockram CS, et al. Prediction of hypertension, diabetes, dyslipidaemia or albuminuria using simple anthropometric indexes in Hong Kong Chinese. Int J Obes Relat Metab Disord. 1999;23:1136–1142. doi: 10.1038/sj.ijo.0801043. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organisation, International Association for the Study of Obesity, International Obesity TaskForce. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health, Communications; 2000. [Google Scholar]
- 33.Kanazawa M, Yoshiike N, Osaka T, et al. Criteria and classification of obesity in Japan and Asia-Oceania. Asia Pac J Clin Nutr. 2002;11(Suppl 8):S732–S737. doi: 10.1159/000088200. [DOI] [PubMed] [Google Scholar]
- 34.Zhou BF Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 35.Ko GT, Tang J, Chan JC, et al. Lower BMI cut-off value to define obesity in Hong Kong Chinese: an analysis based on body fat assessment by bioelectrical impedance. Br J Nutr. 2001;85:239–242. doi: 10.1079/bjn2000251. [DOI] [PubMed] [Google Scholar]
- 36.Yajnik CS, Yudkin JS. The Y-Y paradox. Lancet. 2004;363:163. doi: 10.1016/S0140-6736(03)15269-5. [DOI] [PubMed] [Google Scholar]
- 37.Ko GT, Tang JS. Waist circumference and BMI cut-off based on 10-year cardiovascular risk: evidence for ‘central pre-obesity’. Obesity (Silver Spring) 2007;15:2832–2839. doi: 10.1038/oby.2007.336. [DOI] [PubMed] [Google Scholar]
- 38.Ferland M, Despres JP, Tremblay A, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr. 1989;61:139–148. doi: 10.1079/bjn19890104. [DOI] [PubMed] [Google Scholar]
- 39.Koester RS, Hunter GR, Snyder S, et al. Estimation of computerized tomography derived abdominal fat distribution. Int J Obes Relat Metab Disord. 1992;16:543–554. [PubMed] [Google Scholar]
- 40.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 41.Eston RG, Fu F, Fung L. Validity of conventional anthropometric techniques for predicting body composition in healthy Chinese adults. Br J Sp Med. 1995;29:52–56. doi: 10.1136/bjsm.29.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Waart FG, Li R, Deurenberg P. Comparison of body composition assessments by bioelectrical impedance and by anthropometry in premenopausal Chinese women. Br J Nutr. 1993;69:657–664. doi: 10.1079/bjn19930067. [DOI] [PubMed] [Google Scholar]
- 43.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 44.Szeto CC, Chow KM, Kwan BC, et al. New-onset hyperglycemia in nondiabetic Chinese patients started on peritoneal dialysis. Am J Kidney Dis. 2007;49:524–532. doi: 10.1053/j.ajkd.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 45.de Simone G, Devereux RB, Chinali M, et al. Strong Heart Study Investigators. Prognostic impact of metabolic syndrome by different definitions in a population with high prevalence of obesity and diabetes: the strong heart study. Diabetes Care. 2007;30:1851–1856. doi: 10.2337/dc06-2152. [DOI] [PubMed] [Google Scholar]
- 46.Athyros VG, Ganotakis ES, Elisaf MS, et al. GREECE-METS Collaborative Group. Prevalence of vascular disease in metabolic syndrome using three proposed definitions. Int J Cardiol. 2007;117:204–210. doi: 10.1016/j.ijcard.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 47.Sandhofer A, Iglseder B, Paulweber B, et al. Comparison of different definitions of the metabolic syndrome. Eur J Clin Invest. 2007;37:109–116. doi: 10.1111/j.1365-2362.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 48.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National health and nutrition examination survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 49.Thomas GN, Ho SY, Janus ED, et al. The US National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III) prevalence of the metabolic syndrome in a Chinese population. Diabetes Res Clin Pract. 2005;67:251–257. doi: 10.1016/j.diabres.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 51.Ko GT, So WY, Chan NN, et al. Prediction of cardiovascular and total mortality in Chinese type 2 diabetic patients by the WHO definition for the metabolic syndrome. Diabetes Obes Metab. 2006;8:94–104. doi: 10.1111/j.1463-1326.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- 52.Weir MR. The role of combination antihypertensive therapy in the prevention and treatment of chronic kidney disease. Am J Hypertens. 2005;18(Pt 2):100S–105S. doi: 10.1016/j.amjhyper.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka H, Shiohira Y, Uezu Y, et al. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006;69:369–374. doi: 10.1038/sj.ki.5000050. [DOI] [PubMed] [Google Scholar]
- 54.Chen J, Gu D, Chen CS, et al. Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrol Dial Transplant. 2007;22:1100–1106. doi: 10.1093/ndt/gfl759. [DOI] [PubMed] [Google Scholar]
- 55.Johnson DW, Armstrong K, Campbell SB, et al. Metabolic syndrome in severe chronic kidney disease: prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton) 2007;12:391–398. doi: 10.1111/j.1440-1797.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 56.Young DO, Lund RJ, Haynatzki G, et al. Prevalence of the metabolic syndrome in an incident dialysis population. Hemodial Int. 2007;11:86–95. doi: 10.1111/j.1542-4758.2007.00158.x. [DOI] [PubMed] [Google Scholar]
- 57.Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 58.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 59.Li PK, Chow KM. The clinical and epidemiological aspects of vascular mortality in chronic peritoneal dialysis patients. Perit Dial Int. 2005;25(Suppl 3):S80–S83. [PubMed] [Google Scholar]
- 60.Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453–1459. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 61.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 62.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 63.Hu FB, Willett WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 64.Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979;32:563–576. doi: 10.1016/0021-9681(79)90119-x. [DOI] [PubMed] [Google Scholar]
- 65.Stevens J, Cai J, Evenson KR, et al. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–841. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 66.Stevens J, Plankey MW, Williamson DF, et al. The body mass index-mortality relationship in white and African American women. Obes Res. 1998;6:268–277. doi: 10.1002/j.1550-8528.1998.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 67.Johansen KL, Young B, Kaysen GA, et al. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80:324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 68.Snyder JJ, Foley RN, Gilbertson DT, et al. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64:1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 69.Szeto CC, Wong TY, Leung CB, et al. Importance of dialysis adequacy in mortality and morbidity of chinese CAPD patients. Kidney Int. 2000;58:400–407. doi: 10.1046/j.1523-1755.2000.00179.x. [DOI] [PubMed] [Google Scholar]
- 70.Wong JS, Port FK, Hulbert-Shearon TE, et al. Survival advantage in Asian American end-stage renal disease patients. Kidney Int. 1999;55:2515–2523. doi: 10.1046/j.1523-1755.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- 71.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States renal data system dialysis morbidity and mortality wave II study. Kidney Int. 2004;65:597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 72.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among ‘large’ ESRD patients in the United States. Kidney Int. 2004;65:2398–2408. doi: 10.1111/j.1523-1755.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 73.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol. 2003;14:2894–2901. doi: 10.1097/01.asn.0000091587.55159.5f. [DOI] [PubMed] [Google Scholar]
- 74.McDonald SP, Collins JF, Rumpsfeld M, et al. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int. 2004;24:340–346. [PubMed] [Google Scholar]
- 75.Johnson DW, Mudge DW, Sturtevant JM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int. 2003;23:276–283. [PubMed] [Google Scholar]
- 76.Ramkumar N, Pappas LM, Beddhu S. Effect of body size and body composition on survival in peritoneal dialysis patients. Perit Dial Int. 2005;25:461–469. [PubMed] [Google Scholar]
- 77.Stompor T, Sulowicz W, Dembinska-Kiec A, et al. An association between body mass index and markers of inflammation: is obesity the proinflammatory state in patients on peritoneal dialysis? Perit Dial Int. 2003;23:79–83. [PubMed] [Google Scholar]
- 78.Holmes CJ, Shockley TR. Strategies to reduce glucose exposure in peritoneal dialysis patients. Perit Dial Int. 2000;20(Suppl 2):S37–S41. [PubMed] [Google Scholar]
- 79.Szeto CC, Chow KM, Leung CB, et al. Increased subcutaneous insulin requirements in diabetic patients recently commenced on peritoneal dialysis. Nephrol Dial Transplant. 2007;22:1697–1702. doi: 10.1093/ndt/gfl834. [DOI] [PubMed] [Google Scholar]
- 80.Lameire N, Matthys D, Matthys E, et al. Effects of long-term CAPD on carbohydrate and lipid metabolism. Clin Nephrol. 1988;30(Suppl 1):S53–S58. [PubMed] [Google Scholar]
- 81.Kurtz SB, Wong VH, Anderson CF, et al. Continuous ambulatory peritoneal dialysis. Three years’ experience at the Mayo clinic. Mayo Clin Proc. 1983;58:633–639. [PubMed] [Google Scholar]
- 82.Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the diabetes prevention program randomized trial. Ann Intern Med. 2005;142:611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martens FM, Visseren FL, Lemay J, et al. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002;62:1463–1480. doi: 10.2165/00003495-200262100-00004. [DOI] [PubMed] [Google Scholar]
- 84.Wong TY, Szeto CC, Chow KM, et al. Rosiglitazone reduces insulin requirement and C-reactive protein levels in type 2 diabetic patients receiving peritoneal dialysis. Am J Kidney Dis. 2005;46:713–719. doi: 10.1053/j.ajkd.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 85.Marshall J, Jennings P, Scott A, et al. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS) Kidney Int. 2003;64:1480–1486. doi: 10.1046/j.1523-1755.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 86.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint National committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 87.Li PK, Chow KM, Wong TY, et al. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med. 2003;139:105–112. doi: 10.7326/0003-4819-139-2-200307150-00010. [DOI] [PubMed] [Google Scholar]
- 88.Despres JP, Lemieux I, Prud’homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ. 2001;322:716–720. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Axelsson J, Rashid Qureshi A, Suliman ME, et al. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr. 2004;80:1222–1229. doi: 10.1093/ajcn/80.5.1222. [DOI] [PubMed] [Google Scholar]
- 90.Axelsson J, Møller HJ, Witasp A, et al. Changes in fat mass correlate with changes in soluble sCD163, a marker of mature macrophages, in patients with CKD. Am J Kidney Dis. 2006;48:916–925. doi: 10.1053/j.ajkd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 91.Hollis J, Corden E, Williams PF. Longitudinal evaluation of a weight reduction program for patients on peritoneal dialysis. Perit Dial Int. 2005;25(Suppl 3):S152–S154. [PubMed] [Google Scholar]
- 92.Rucker D, Padwal R, Li SK, et al. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolfson M, Piraino B, Hamburger RJ, et al. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. 2002;40:1055–1065. doi: 10.1053/ajkd.2002.36344. [DOI] [PubMed] [Google Scholar]
- 94.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 95.Isbel NM, Haluska B, Johnson DW, et al. Increased targeting of cardiovascular risk factors in patients with chronic kidney disease does not improve atheroma burden or cardiovascular function. Am Heart J. 2006;151:745–753. doi: 10.1016/j.ahj.2005.06.017. [DOI] [PubMed] [Google Scholar]