Introduction
Hereditary renal failure includes a wide spectrum of diseases, ranging from the well-known autosomal-dominant polycystic kidney disease (ADPKD) to rare syndromes. The latter group includes a broad variety of diseases, often with a whole host of extra-renal manifestations. Many of these syndromes are little known among adult nephrologists, be it simply because they are rare, or due to the fact that their names are difficult to remember. Most of these rare hereditary disorders carry an eponym, and it is not uncommon to see several people immortalized in the name of a single disease. Finally, several different eponyms may be in concurrent use, despite the fact that a pathogenetic or genetic classification is already available. Knowledge of these rare genetic syndromes may help in clinical practice, and criteria for a clinical diagnosis often exist. Using these criteria, the clinician can frequently make an instant clinical diagnosis, which may have profound implications for clinical management as well as for the relatives of the patient and lead to genetic testing, if appropriate. One may argue that this is more or less the business of paediatricians and paediatric nephrologists but that is not entirely true. Some of these patients may attend adult renal clinics for years or even decades [1] under the label of ‘chronic renal failure’, until the correct diagnosis is made, often by someone who has encountered the disease before. Furthermore, all of these patients grow older and adult nephrologists may encounter them on dialysis, for transplant evaluation or post-transplant care. We present two cases of the same hereditary renal disease with many ‘extra-renal’ symptoms and with considerable eponymous confusion. We discuss clinical features, diagnostic approach and pathogenesis of this disorder. We also provide some insight into the biographies of the four people whose names are immortalized in the eponyms under discussion.
Cases
The first patient was a 22-year-old man on peritoneal dialysis who was referred for evaluation regarding a third renal transplant. He had developed end-stage renal failure with polyuria due to renal dysplasia at the age of 9 years. Since then, he had two failed cadaveric renal transplants and resumed dialysis since 2005. He had mental retardation as well as visual impairment due to rod–cone dystrophy. On examination he was well, with a neat Tenckhoff catheter in situ. His hands looked normal with five normal-sized fingers on each hand; he was slim. His brother was with him in the clinic. He, too, had mental retardation and visual impairment. The letters listed several different diagnoses. On offer were Laurence–Moon–Bardet–Biedl syndrome, Bardet–Biedl syndrome and, finally, Laurence–Moon–Biedl syndrome. The consultant thought long and hard and murmured something about polydactyly and obesity. While he could not come up with a quick solution to this eponymous confusion, he remembered another, slightly different case he had encountered a few years earlier.
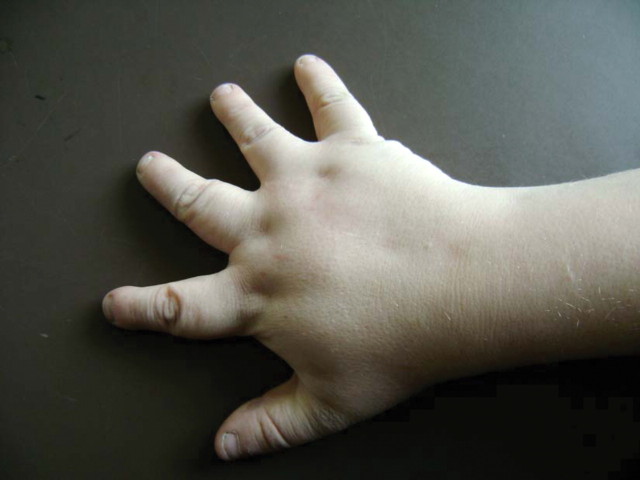
The second patient was a 29-year-old man who had reached end-stage renal disease at age 14 and who now attended a transplant follow-up clinic. He had received a first cadaveric renal transplant at age 14 after 9 months of peritoneal dialysis, and transplant function was good. On the first encounter with this man, three findings were very obvious: first, the patient was obese with 136.5 kg at 185 cm, giving him a body mass index of 39.88. Second, he was legally blind due to retinal dystrophy. Third, shaking hand with the patient revealed that each hand possessed five quite short stubby fingers (Figure 1). According to the notes, he had been born with six fingers on each hand and a sixth toe on one foot. The supernumerary fingers and toes had been removed surgically at 2 years of age. Further examination showed hypospadia, and previous letters mentioned hypogonadism. He had five toes on each foot and a scar after removal of a supernumerary toe on one foot. He also had mild learning disability and worked in a workshop for the blind. By all standards, he was a cheerful young man, despite all his medical problems. There was no family history at all. Again, different eponyms were proposed although Laurence–Moon–Bardet–Biedl syndrome was commonly used.
Fig. 1.
Right hand of patient 2. Note the short stubby fingers. Note the scars from previous hand surgery. Polydactyly had been present at birth but was corrected surgically at age 2.
What is the correct name of the disease and who were the people behind the eponyms?
Discussion
The first step in solving this problem is to get the syndromes right. Fortunately, there is an accessible and reliable authority to guide us, namely the Online Mendelian Inheritance in Man (OMIM) register. According to OMIM, Bardet–Biedl syndrome (BBS, OMIM 20 990) is an autosomal-recessive condition with a wide spectrum of clinical features [2]. The syndrome is genetically heterogeneous with 14 subtypes, BBS 1 to BBS 14. It is believed to be more common in Arab countries, and prevalence rates as high as 1 in 13 500 have been reported in Kuwait. A similar high incidence has been reported in Newfoundland and a founder mutation is probably responsible. On a worldwide basis, BBS is rare with prevalence rates as low as 1:160 000 in Switzerland. Most cases are diagnosed in childhood. The male to female ratio is 1.3:1 while the reason for this imbalance remains unknown. Clinical features include rod–cone dystrophy and visual impairment, developmental delay, polydactyly and/or brachydactyly, obesity and renal failure. The renal manifestation resembles nephronophthisis, with polyuria, cortico-medullary cysts and decline in renal function.
Laurence–Moon syndrome (LMS) also exists in OMIM and carries the number 245 800. The features in the four sibs reported by Laurence and Moon in 1866 [3] and later by others were mental retardation, pigmentary retinopathy, hypogenitalism and spastic paraplegia. However, currently it is no longer believed that LMS exists as a separate entity [4]. Findings in a large survey of Bardet–Biedl syndrome in Newfoundland underpin this assessment. In this study, Moore and co-workers found two patients previously diagnosed as having LMS [5]: one patient turned out to be from a consanguineous pedigree with linkage to the BBS 5 gene, and the other was a patient with BBS 6. The authors concluded that BBS and LMS are not distinct [5].
Back to the two patients and it is time for a clinical diagnosis. Beales and co-workers have proposed criteria for a clinical diagnosis of BBS (Table 1). Case 1 fulfils three primary and two secondary criteria, thus permitting a clinical diagnosis of BBS [6]: he had rod–cone dystrophy, learning disabilities and renal involvement as primary features and developmental delay as well as polyuria as secondary features. The clinical diagnosis in case 1 would thus be Bardet–Biedl syndrome. We were initially puzzled by the normal weight. However, in Beales’ and colleagues’ seminal series, only 52% of patients with BBS were obese while 72% were overweight. Hence, obesity is not a prerequisite for diagnosis. The normal fingers in case 1 also confused us. While polydactyly or brachydactyly are typical features of BBS, they are by no means uniformly present. In Beales’ series, 69% of patients with BBS were born with accessory digits. Of note, toes need to be examined in search of polydactyly. Case 2 is even easier to diagnose when Table 1 is used. This patient had rod–cone dystrophy, polydactyly, obesity, hypogonadism, learning disabilities and renal involvement (six primary features where only four are required) as well as brachydactyly as a secondary feature. Here, too, a clinical diagnosis of Bardet–Biedl syndrome can be made on the spot.
Table 1.
Diagnostic criteria for Bardet–Biedl syndrome [6]; four primary features are required or, alternatively, three primary features and two secondary features to permit a clinical diagnosis
| Primary features | |
| Rod–cone dystrophy | |
| Polydactyly | |
| Obesity | |
| Learning disabilities | |
| Hypogonadism in males | |
| Renal anomalies | |
| Secondary features | |
| Speech disorder/delay | |
| Strabismus/cataracts/astigmatism | |
| Brachydactyly/syndactyly | |
| Developmental delay | |
| Polyuria/polydipsia (nephrogenic diabetes insipidus) | |
| Ataxia/poor coordination/imbalance | |
| Mild spasticity (especially lower limbs) | |
| Diabetes mellitus | |
| Dental crowding/hypodontia/small roots/high arched palate | |
| Left ventricular hypertrophy/congenital heart disease | |
| Hepatic fibrosis |
The pathogenesis of BBS is also interesting [7,8]. Nachury and colleagues were the first to demonstrate that BBS proteins co-localize with cilia and proposed a defect in vesicular transport to the cilium [9]. Cilia and flagella are ancient cell organelles that perform diverse biological roles, such as locomotion, chemo-, mechano-, and photosensation and reproduction. Data from C. elegans studies suggest that loss-of-function mutations in the genes of C. elegans homologous to BBS7 and BBS8 compromise cilia structure and function. It is currently not quite clear how a defect in the cilia causes all clinical features of BBS. The link to obesity seemed particularly difficult to explain by a cilia dysfunction. However, very recent research has shown that a typical primary cilium is present in differentiating preadipocytes [10]. Of note, BBS is only one disease within a whole spectrum of diseases with dysfunctional cilia and the term ‘ciliopathies’ has been coined [7]. The term encompasses Alström syndrome, BBS, Meckel syndrome, primary ciliary dyskinesis and the nephronophthisis complex (Table 2). It is important to remember that these ciliopathies share the same peculiar type of renal manifestation, namely the nephronophthisis phenotype. Imaging will show normal-sized kidneys with numerous cysts at the cortico-medullary junction or in the medulla. The clinical course is also peculiar in that polyuria is very common. Finally, unlike in many other forms of renal disease, hypertension is usually absent. ADPKD is sometimes viewed as a ciliopathy (the ‘ciliary hypothesis’), but the disease is beyond the scope of this little article. It also behaves differently from the other ciliopathies in that it is autosomal dominant while the others are autosomal recessive. ADPKD disease is reviewed in great detail elsewhere [11], as is the ciliary hypothesis of the disease.
Table 2.
The ciliopathies as noted in the OMIM database; note that inheritance of all diseases is autosomal-recessive
| Disease | Clinical features | Gene | OMIM number |
|---|---|---|---|
| Alström syndrome | Childhood obesity and type II diabetes, blindness due to congenital retinal dystrophy, and sensori-neural hearing loss | ALMS1 gene, 2p13 | 203800 |
| Bardet-Biedl syndrome | Rod-cone dystrophy | BBS1, 11q13 | 209901 |
| Polydactyly | BBS2, 16q21 | 606151 | |
| Obesity | BBS3, 3p12-q13 | 608845 | |
| Learning disabilities | BBS4, 15q22.3 | 600374 | |
| Hypogonadism in males | BBS5, 2q31 | 603650 | |
| Renal anomalies (similar to nephronophthisis) | BBS6, 20p12 | 604896 | |
| BBS7, 4q27 | 607590 | ||
| BBS8, 14q32.11 | 608132 | ||
| See also text and table 1 | BBS9, 7p14 | 607968 | |
| BBS10, 12q | 610148 | ||
| BBS11, 9q33.1 | 602290 | ||
| BBS12, 4q27 | 610683 | ||
| BBS13, 17q23 | 609883 | ||
| BBS14, 12q21.3 | 610142 | ||
| Nephronophthisis | Renal cysts in the cortico-medullary junction with polyuria and | NPHP1, 2q13 | 256100 |
| renal impairment | NPHP2, 9q31 | 602088 | |
| Other manifestations: | NPHP3, 3q22 | 604387 | |
| Tapeto-retinal degeneration (Senior Løken syndrome) | NPHP4, 1p36 | 606966 | |
| Hepatic fibrosis | NPHP5, 3q21.1 | 609254 | |
| Cone shaped epiphyses | NPHP6, 12q21.3 | 610142 | |
| Vermis aplasia and ataxia (Joubert syndrome) | NPHP7, 16p13.3 | 611498 | |
| Primary ciliary diskinesis | Sinusitis, bronchiectasis, situs inversus (Kartagener's syndrome) | 9p21-p13 | 24440 |
| Meckel syndrome (also known as Meckel Gruber syndrome) | Dysplastic kidneys, polydactyly, occipital encephalocele; high mortality; prenatal ultrasound diagnosis possible | MKS1, 17q21–q24 | 249000 |
| MKS2, 11q13 | 603194 | ||
| MKS3, 8q24 | 607361 |
We have now appreciated that our patients both have BBS and that Laurence–Moon syndrome does not exist. More confusion has been caused by the fact that other eponyms have been used. OMIM and current evidence suggest that these are obsolete as well. Historically, Laurence–Moon–Bardet–Biedl syndrome has been most common, but Laurence–Biedl syndrome has been used as well. Of note, a PubMed search for Laurence–Moon–Bardet–Biedl syndrome still yields 159 articles, some of which have recently been published in prestigious journals. The term is also still in use in Wikipedia. Misspellings take a further toll, and even a PubMed search for ‘Biedle syndrome’ still yields five results. Table 2 also illustrates the ‘eponymophilia’ [12] in the field of the ciliopathies, and it may be helpful to cite the OMIM number, if possible, to avoid confusion.
Who were all of these people whose names are immortalized in Bardet–Biedl syndrome? All we know about French physician Georges Louis Bardet is that he was born in 1885. In his graduation thesis at the University of Paris in 1920, Bardet wrote about a medical condition characterized by obesity, retinitis pigmentosa, polydactyly and hypogonadism. Even the date of Bardet's death is unknown, and we are none the wiser after an extensive Internet search through Google, PubMed and Whonamedit.com. A photograph of this man does not exist. Maybe it is just high time for a young nephrologist or endocrinologist to dig into the archives and explore the biography of George Louis Bardet.
We know a bit more about the second person in this group of four: Arthur Biedl (Figure 2) was born on 4 October 1869 in Kiskomlos in Hungary (nowadays Comlosu Mic, Romania) and died on 26 August 1933 in Austria. Biedl was a famous Hungarian pathologist of the 19th century. In 1913, Biedl was offered the chair of experimental pathology at the German University of Prague. He, however, was not clinically oriented and left the beds in the care of his colleague Julius Riehl, a cardiologist. He is considered one of the fathers of endocrinology and published the first textbook in this field [13]. Incidentally, he also proved that humans need their adrenal glands to survive. He published the cases of two children with what we now know as BBS in 1922.
Fig. 2.

Arthur Biedl (1869–1933), courtesy of Prof. P. Beales, University College London.
Some interesting facts are also known about John Zachariah Laurence (1829–70, Figure 3). Laurence was an eminent 19th-century ophthalmologist, founder of the Royal Eye Hospital and founding editor of Ophthalmic Review, the first major English journal devoted to ophthalmology. Another piece of his legacy is omnipresent to the present day: Laurence introduced a modified ophthalmoscope in Britain after studies of refraction in Utrecht some years before. Laurence died at the young age of 42 [14].
Fig. 3.
John Zachariah Laurence (1829–70), courtesy of Prof. P. Beales, University College London.
Of the four people discussed in this article, Robert Charles Moon (1844–1914, Figure 4) has the most interesting biography by far. His father, William Moon (1818–94), had lost his sight in early adolescence aged 22. William Moon acquired fame through his invention of Moon type, an embossed reading for the blind—a competitor to Braille's method. Braille is cheap and simple to produce but difficult to learn, while the opposite is true of Moon type. It is believed that young Robert Moon helped his father in translating and transcribing reading matter for the visually handicapped. His initial plans were to become a priest but, for whatever reason, that plan was later abandoned. It is assumed that early exposure to his father's work for the blind made Robert interested in ophthalmology. Moon qualified in medicine in London and later worked at South London Ophthalmic Hospital where he published, in 1866, the first description of what we now know as BBS together with his senior colleague John Zachariah Laurence. Robert C. Moon left for the United States where he settled into ophthalmological practice in Philadelphia. He carried on his fathers work, establishing the Moon Press for the Blind and involving himself in their welfare. Following his father's example, Robert C. Moon continued his philanthropic activities for the blind even after his retirement.
Fig. 4.

Robert Charles Moon (1845–1914), courtesy of Prof. P. Beales, University College London.
The use of eponyms has seen some controversial discussion in recent years, and the issue is well beyond the scope of this little article. Suffice to say that some want to do away with eponyms altogether [15] in favour of a descriptive nomenclature. They argue that some eponyms bear the names of individuals with a shady past, other carry different names in different countries and yet others do not honour the people who discovered the disease. In this case, the eponym BBS immortalizes two people who reported the disease decades after the initial description by Moon and Laurence. Others argue that eponyms add colour to medicine and that a total revision of the nomenclature would be impractical [16]. Regardless of our individual opinion on this matter, we should probably ask more questions about the men and women behind the eponyms. This may indeed provide interesting insight and add historic flavour to our daily work.
Teaching points
Bardet–Biedl syndrome (BBS, OMIM 20990) is an autosomal-recessive disease with many clinical features. A set of diagnostic criteria exists to facilitate a clinical diagnosis.
BBS is genetically heterogeneous and 14 different genes, BBS1 to BBS 14, have been identified.
BBS is a ciliopathy, together with Alström syndrome, primary ciliary dyskinesis, Meckel syndrome and the nephronophthisis group.
Asking questions about the names behind the eponyms can add historic flavour to our daily work.
Acknowledgments
We are indebted to Professor P. Beales, University College London, for Figures 2–4.
Conflict of interest statement. None declared.
References
- 1.Saif A, Woywodt A, Coward RA. No eye for ears. Nephrol Dial Transplant Plus. 2009;2:173–174. doi: 10.1093/ndtplus/sfn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardet–Biedl Syndrome (OMIM 209900) National Institutes of Health Online Mendelian Inheritance in Man. 2009. http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=209900. (22 February 2009, date last accessed)
- 3.Laurence JZ, Moon RC. Four cases of retinitis pigmentosa occurring in the same family and accompanied by general imperfection of development. Ophthal Rev. 1886;2:32–41. doi: 10.1002/j.1550-8528.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 4.Laurence–Moon Syndrome (OMIM 245800) National Institutes of Health Online Mendelian Inheritance in Man. 2009. http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=245800. (27 February 2009, date last accessed)
- 5.Moore SJ, Green JS, Fan Y, et al. Clinical and genetic epidemiology of Bardet–Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A. 2005;132:352–360. doi: 10.1002/ajmg.a.30406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beales PL, Elcioglu N, Woolf AS, et al. New criteria for improved diagnosis of Bardet–Biedl syndrome: results of a population survey. J Med Genet. 1999;36:437–446. [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 8.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet–Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 10.Marion V, Stoetzel C, Schlicht D, et al. Transient ciliogenesis involving Bardet–Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci USA. 2009;106:1820–1825. doi: 10.1073/pnas.0812518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 12.Matteson EL, Woywodt A. Eponymophilia in rheumatology. Rheumatology (Oxford) 2006;45:1328–1330. doi: 10.1093/rheumatology/kel259. [DOI] [PubMed] [Google Scholar]
- 13.Biedl A. The Internal Secretory Organs: Their Physiology and Pathology. London: John Bale, Sons and Danielsson; 1913. [Google Scholar]
- 14.Sorsby A. Zachariah Laurence: a belated tribute. Br J Ophthalmol. 1932;16:727. doi: 10.1136/bjo.16.11.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woywodt A, Matteson E. Should eponyms be abandoned? Yes. BMJ. 2007;335:424. doi: 10.1136/bmj.39308.342639.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitworth JA. Should eponyms be abandoned? No. BMJ. 2007;335:425. doi: 10.1136/bmj.39308.380567.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]