Abstract
Management of chronic kidney disease–mineral bone disorder can be difficult in renal patients. This review aims to explain why the control of disturbed calcium, phosphate, parathyroid hormone and vitamin D metabolism is important in dialysis patients. The methods available to regulate these parameters include diet, phosphate binders, dialysate calcium, native vitamin D, active vitamin D derivatives and calcimimetics. An overview of current treatment guidelines will be discussed.
Keywords: calcium, phosphate, PTH, uraemia, vitamin D
Introduction
The management of calcium and phosphate metabolism in renal patients is a common problem that has been challenging nephrologists for many years. The bone mineral metabolism abnormalities that occur in renal disease are now encompassed in the term chronic kidney disease–mineral bone disorder (CKD–MBD) [1]. This term also includes ectopic calcification and bone abnormalities.
Compared to the general population, renal patients have an increased mortality rate [2], and ∼50% is attributable to cardiovascular causes [3]. This increased mortality may, in part, be due to the presence of one or more factors of CKD–MBD. This review will try to answer the questions why the control of calcium and phosphate is important in dialysis patients and how this can be achieved. The evidence for and difficulties of the various available management options will also be covered.
Methods
To perform this review, a structured search in PubMed was undertaken. All aspects of CKD–MBD were searched in the context of renal disease and/or dialysis. Medications discussed were also reviewed in the context of renal disease and/or dialysis. Further, separate searches were performed on calcium, phosphate, phosphorus, vitamin D and vascular calcification.
Search terms
The search terms were CKD–MBD, phosphate, phosphorus, calcium, parathyroid hormone, renal disease, chronic kidney disease, haemodialysis, vascular calcification, renal osteodystrophy, renal bone disease, adynamic bone disorder, vitamin D, calcitriol, alphacalcidol, paracalcitol, 25 vitamin D, 1,25 vitamin D, phosphate binder, calcium containing phosphate binder, fosrenol/lanthanum carbonate, renagel/sevelamer, calcium acetate and calcium carbonate.
CKD–MBD
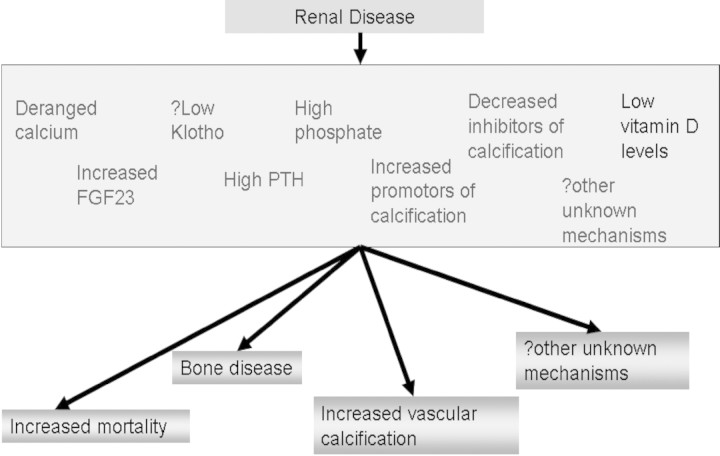
CKD–MBD is a term describing a spectrum of abnormalities occurring in renal impairment and includes vascular and soft tissue calcification, disorders of bone turnover and derangements of bone mineral metabolism [1]. The increasing interest and research in this area has led to a greater understanding of the underlying mechanisms regulating these processes. A diagrammatic representation of potential important factors is shown in Figure 1.
Fig. 1.
Potential factors important in outcome of renal patients.
Simplistically, as renal disease progresses, secondary hyperparathyroidism occurs due to the inability to remove phosphate and loss of 1-alpha hydroxylase activity in the kidney leading to a lower serum calcium. These changes lead to positive feedback mechanisms at the parathyroid gland increasing the level of parathyroid hormone (PTH).
Raised PTH is required to some extent to maintain normal bone turnover as there is skeletal resistance to the hormone in renal disease [4]. Lower levels of PTH are typical of the adynamic bone disorder (ABD) that has been associated with the overuse of calcium phosphate binders and active vitamin D therapy [5,6]. It is, however, unlikely that this is the major cause of ABD: patients with this condition tend to be older, malnourished and have multiple comorbidities; their requirements for phosphate binders and active vitamin D therapy to suppress PTH are lower. It is more likely that ABD is a marker at the bone level of the malnutrition–inflammation–atherosclerosis (MIA) syndrome [7]. However, the type of bone disease found in patients with either high or low bone turnover, osteomalacia or osteopenia could not be correlated to PTH levels in all patients [8]. The Kalantar–Zadeh study [9] showed a strong association between incremental PTH values and death risk above PTH levels of 300 ng/l that was significant above 400 ng/l. If correction is made for age, comorbidity and albumin, the relationship between PTH and mortality is positive and linear [9].
The case for calcidiol
Native vitamin D precursors are initially ingested or formed in the skin secondary to exposure to UVB light, in the form of D3 (cholecalciferol). D2 (ergocalciferol) is a commonly used artificial dietary substitute. D2 or D3 is then 25-hydroxylated in the liver to 25-hydroxycholecalciferol (calcidiol) and stored until required when the highly regulated 1-alpha hydroxylation occurs to form the active 1,25-dihydroxycholecalciferol (calcitriol). Renal patients are known to have both calcidiol and calcitriol deficiencies that develop over the course of CKD [10] and are highly prevalent in dialysis populations [11,12]. While normalization of 25-hydroxyvitamin levels to a level >75 nmol/l (>30 μg/l) is recommended for healthy individuals and patients with CKD stages 3 and 4 [13], supplementation in CKD stage 5 has traditionally not been considered indicated, due to reduced or absent hydroxylation to calcitriol. There are a number of reasons why this attitude may be erroneous. Investigations [11,14] show that calcidiol and calcitriol concentrations are correlated even in severe uraemia, suggesting that increased substrate will help to normalize calcitriol activity. Furthermore, vitamin D receptors are widely distributed throughout the body, and extra-renal 1-alpha hydroxylase activity is found in several cells including bone [15], macrophages [16], endothelium, prostate, breast and brain [17]. In the presence of uraemia, extra-renal production of calcitriol is upregulated [17,18]. Vitamin D deficiency is associated with a wide variety of diseases including several cancers, type 2 diabetes mellitus, pneumonia, tuberculosis, multiple sclerosis, inflammatory bowel disease, rheumatoid arthritis, hypertension and atherosclerosis [18]. A recent randomized controlled trial has demonstrated a protective effect of cholecalciferol against cancer [19], and low levels of 25-hydroxycholecalciferol are associated with excess mortality in dialysis [20]. Cholecalciferol supplementation has been shown to reduce PTH in CKD stage 3 [21] but has little or no effect in stages 4 and 5 [22–24]. However, a recent article has shown that the combination of cholecalciferol and calcium in post-menopausal women can reduce PTH in moderate CKD (stages 3 and 4); patients with severe renal impairment were excluded [25]. In conclusion, D3 or D2 supplementation may be advantageous, both by stimulating 1,25-(OH)D production even in severe uraemia and in providing a substrate for extra-renal effects independent of renal function and renal 1-alpha hydroxylase activity. Caution mandates that normalization of calcidiol levels should also be a goal for CKD stage 5 patients: if it works, it may help prevent atherosclerosis, hypertension, immune deficiency and cancer; if it does not, there is no harm done.
The hypovitamin state of calcitriol found in CKD may also be exacerbated by an increase in levels of fibroblast growth factor 23 (FGF23). FGF23 is now thought to be the main regulator of phosphate homeostasis and its levels rise early in CKD prior to any derangement of phosphate is observed [26]. However, FGF23 has been shown to decrease the activity of 1-alpha hydroxylase leading to less formation of active vitamin D [27]. FGF23 is formed in bone but acts at the kidney, and it has now been associated with mortality in renal disease, independent of phosphate levels; the mechanism for this association remains, however, unknown [28].
CKD–MBD: a vascular disease
The other main component of CKD–MBD is calcification. Renal patients are prone to developing medial calcification of their vasculature in addition to intimal calcification that is more commonly associated with atherosclerosis. Vascular calcification has been associated with increased phosphate and ingested calcium load [29] and has also been shown to predict mortality [30]. Since hypercalcaemia and hyperphosphataemia are independently associated with cardiovascular death in CKD [9,10], a simplistic interpretation is that an increase in the calcium × phosphate product leads to increased vascular deposition of calcium phosphate, leading to arteriosclerosis, a ‘true’ hardening of the arteries. However, vascular calcification is now known to be an actively regulated process involving several factors [31]. The medial calcification is hypothesized to be due to phenotypic changes of vascular smooth muscle cells. These cells are under strict regulation from a variety of factors including fetuin A and matrix Gla proteins that become deranged as renal impairment progresses [32]. Hyperphosphataemia leads to the formation of osteoblast-like cells in the vessel wall [33], and deposition of calcium phosphate crystals induces cell death in vascular smooth muscle cells [34].
The medial calcification that develops restricts the dilatation of the artery, and hence no longer allows the expansile properties of the artery to absorb the pressure variation through the cardiac cycle. This is described as vascular stiffness that has also been associated with increased mortality in dialysis patients [35,36].
Although calcium and phosphate regulation is thus an integral part of the management of all the complications and components of CKD–MBD, it is not the only answer. The primary objectives are the avoidance of hyperphosphataemia, hypercalcaemia and also hypocalcaemia with its associated risks of cardiac arrhythmias and hyperparathyroidism. Which treatment to use is more debatable due to the interactions with bone and vascular complications, the latter will be discussed in detail in the following sections.
Phosphate control
The first-line therapy for managing increasing serum phosphate levels is the initiation of a phosphate-restricted diet. However, many patients, particularly the young, find this restrictive and interfering with their normal lives. Phosphate binders are then introduced, and depending on the available budget, there are increasing variations to choose from. Table 1 lists the different phosphate binders currently available with a summary of the benefits and problems with each one.
Table 1.
Summary of phosphate binders used in renal disease
| Phosphate binder | Generic name | Common side effects | Potential disadvantage | Potential benefits |
|---|---|---|---|---|
| Phosex PhosLo | Calcium acetate | Nausea Constipation, diarrhoea Hypercalcaemia | Increase calcium load leading to increased calcification | Cost Less calcium than other calcium binders |
| Calcichew Calcium 500 Titralac | Calcium carbonate | Nausea Constipation, diarrhoea Hypercalcaemia | Increase calcium load leading to increased calcification | Cost |
| OsvaRen | Calcium acetate + Magnesium carbonate | Nausea Constipation, diarrhoea Hypercalcaemia Hypermagnasaemia | Lower calcium load than other calcium-containing phosphate binders. Suitable for patients with normo- or hypomagnesaemia, e.g. PD patients | |
| Renagel | Sevelamer hydrochloride | Nausea Indigestion | Expensive Acidosis in pre-dialysis Multiple tablets—compliance poor | Attenuates progression of calcification compared to calcium binders Mortality benefit in over 65 years Lowers LDL cholesterol |
| Fosrenol | Lanthanum carbonate | Nausea Diarrhoea | Metal and long term effects unknown | One tablet only per meal increasing compliance |
| Alucaps | Aluminium hydroxide | Aluminium toxicity including encephalopathy and bone disease | Excellent phosphate binder |
Historically, aluminium hydroxide and calcium-containing binders were used to control phosphate. However, aluminium is now known to accumulate with prolonged exposure and causes severe toxicity including encephalopathy, myopathy and decrease in bone turnover and is therefore no longer recommended for long-term or regular use. Calcium-containing binders are now the most common first-line phosphate binders used in clinical practice. The ingested calcium load has now been shown to be associated with the presence of increased vascular calcification and ABD [29]. The National Kidney Foundation–Disease Outcomes Quality Initiative guidelines (K/DOQI) [14], published in 2003, recommended a maximum intake of 2 g elemental calcium per day, of which only 1.5 gm should be due to a prescribed phosphate binder (corresponding to 3.7 g calcium carbonate or 6 g calcium acetate tablets daily). This guideline was opinion based as there is no data to suggest that a certain amount of calcium is either safe or detrimental. Secondary to these guidelines, a policy of a more sparing use of calcium binders wherever funding allows was followed. This has lead to an increased use of the alternative, more expensive, calcium-free binders that are now available on the market.
The newer phosphate binders include Renagel® (sevelamer hydrochloride), a resin-based binder and Fosrenol® (lanthanum carbonate), a metal-based binder, neither containing calcium. Renagel has been shown to attenuate the progression of vascular calcification when compared to calcium-based binders [37]. This could be in part due to the lipid lowering effect of Renagel because, when similar lipid profiles are achieved with a statin, no differences were found between calcium binders and Renagel [38]. Both of these trials, however, had differing PTH control between each arm and therefore may not be comparable.
One study suggested a survival benefit with the use of Renagel over calcium binders [39]. However, a large randomized trial (Dialysis Clinical Outcomes Revisited, DCOR) showed no survival advantage in the overall prevalent dialysis population but only a benefit in patients older than 65 years [40]. From the above discussion, one would expect any survival advantage of sevelamer (or lanthanum) to be limited to patients with a tendency to hypercalcaemia; surprisingly, no study analysing this question has yet been published.
Despite these somewhat ambiguous benefits, Renagel does have some disadvantages. The hydrochloride compound can cause acidosis, especially in pre-dialysis patients. This problem should be overcome with the newer carbonate compound that is now licensed in the USA. The Renagel tablets are also large, and some patients have difficulty tolerating them that can lead to reduced compliance.
The other new non-calcium phosphate binder is lanthanum carbonate and involves one tablet to be taken per meal. This regimen was shown to be more palatable to some patients and may improve compliance [41]. However, lanthanum has also potential disadvantages in that it is based on an elemental metal. Due to the previous problems with aluminium, regulatory authorities and nephrologists alike have been guarded of its potential harm. However, lanthanum absorption is very low compared to aluminium and has a hepatic excretory mechanism as compared to aluminium's renal excretion. It has not been shown to cross the blood–brain barrier [42]. Long-term clinical and bone studies are reassuring [43]. Lanthanum is a newer product and as yet no large survival or calcification studies have been published.
A recently introduced product, OsvaRen®, supplements calcium with magnesium carbonate, achieving equivalent phosphate binding at a lower calcium load; it is thus intermediate between calcium-containing and calcium-free phosphate binders. It is particularly suitable for patients with hypomagnesaemia, e.g. peritoneal dialysis patients.
PTH, vitamin D and calcium control
Other compounds used to maintain calcium and phosphate in the recommended target range are vitamin D analogues and calcimimetics. These treatments are mainly used to lower PTH levels, but as calcimimetics reduce calcium and phosphate levels and vitamin D analogues increase them, these drugs are used in different situations or more commonly synergistically.
Calcitriol and 1-alfacalcidol are the traditionally used vitamin D analogues in renal disease providing replacement of the active 1,25-hydroxyvitamin D. They act by restoring a negative feedback to the parathyroid gland, reducing PTH. However, they also act at receptors in the gastrointestinal tract that leads to increased absorption of calcium and phosphate. This can lead to less efficient suppression of PTH as the doses are restricted by hypercalcaemia and hyperphosphataemia. A newer vitamin D analogue, paracalcitol, claims to lead to a lower incidence of these problems. However, in the randomized trial between calcitriol and paracalcitol, the frequency of hypercalcaemia and hyperphosphataemia was similar but paracalcitol lead to less persistent hypercalcaemia [44]. This reduction of persistent hypercalcaemia may still have an impact on morbidity and mortality; one retrospective study has shown a survival advantage in haemodialysis patients over calcitriol [45]. Surprisingly, the excess risk of hypercalcaemia associated with calcitriol may not apply to 1-alfacalcidol: at equivalent doses, intestinal calcium absorption is 80% lower with 1-alfacalcidol treatment compared to calcitriol [46]. No prospective randomized mortality studies have been completed.
Several retrospective studies have shown a survival advantage of active vitamin D supplementation in dialysis [9,47–49], and the advantage is independent of dose. In the study by Teng et al. [47], the effect was equally present in patients with hypocalcaemia, hyperphosphataemia and hypoparathyroidism. This raises the interesting possibility that the excess mortality seen in patients with adynamic bone disease is iatrogenic, due to conventional recommendations to deny these patients therapy with active vitamin D sterols. Similarly, both low and high 1,25(OH)2 vitamin D levels are associated with increased carotid intima–media thickness and calcification in children on dialysis [50]. Thus, the existing epidemiological evidence suggests that all patients with CKD stage 5 should be treated with a small dose of active vitamin D regardless of calcium, phosphate and PTH. However, this does not make the control of calcium and phosphate easier.
Calcimimetics are a novel class of drugs that act at the calcium sensing receptor to reduce PTH secretion. The therapy has the added attraction of lowering serum calcium and serum phosphate and therefore has a role where vitamin D analogues fail. This therapy is only licensed for dialysis patients as it can lead to more severe hypocalcaemia and can increase phosphate in CKD stages 3 and 4 [51]. Animal studies have shown that calcimimetics can halt the formation and even lead to regression of vascular calcification [52]. Prospective trials are in progress that may answer this question in renal patients, and the results should be available in late 2009. A long-term mortality study is also underway. As this medication is expensive, it is only commonly used in the difficult patients whose secondary hyperparathyroidism cannot be controlled with vitamin D analogues alone, and where the alternative is often parathyroidectomy.
Parathyroidectomy is reserved for these patients who have developed an adenoma and/or have tertiary hyperparathyroidism. Associated hypercalcaemia and hyperphosphataemia will usually be cured by the operation. This procedure has been associated with a survival advantage compared to not having a parathyroidectomy, but not to calcimimetic therapy [53]. The use of cinacalcet in these severe patients varies from country to country, and serum iPTH can be lowered with this therapy also.
Changing the calcium content of dialysate can also be utilized to aid calcium control. The pursuit of the K/DOQI guidelines for plasma calcium implies use of a high-calcium dialysate (>1.4 mmol/l) in hypocalcaemic patients and conversely low-calcium dialysate in patients with high/high-normal calcium levels [14].
Using the medications that were discussed, management of each patient can be individually tailored to control their bone chemistry parameters. However, despite the array available, many patients are still not reaching the targets. This may be due to a number of reasons including inadequate phosphate binder efficacy, suboptimal physician attention to the problem and poor patient compliance.
Current guidelines
Many targets have been set over the years and are continuously changing depending on who is advising and on current evidence. The current guidelines available have been summarized in Table 2, and this highlights the variation in ‘targets’ available for the nephrologists to follow. These guidelines claim to be based on evidence but the evidence can be interpreted differently leading to different recommendations. The latest guideline, though this is still under public review and still may undergo changes, is the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines for overall strategy related to CKD–MBD. These guidelines, at least at this provisional stage, show a critical approach to the available evidence and clearly suggest guidance highlighting the lack of evidence in the CKD–MBD field. As unhelpful as this may be in the management of patients, this approach, by highlighting the many areas where evidence is lacking, will hopefully stimulate research.
Table 2.
A comparison of published guidelines for bone mineral metabolism currently available
| Guideline | Year | Calcium | Phosphate | Ca × P product | Parathyroid hormone | Calcium load |
|---|---|---|---|---|---|---|
| KDOQI [14] | 2003 | Normal range preferably 2.1–2.37 mmol/l | 1.13–1.78 mmol/l | <55 mg2/dl2 (4.4 mmol2/l2) | 150–300 pg/ml | 1.5 gm elemental calcium/day as binder |
| UK Renal association [54] | 2007 | Normal range preferably 2.2–2.5 mmol/l | 1.1–1.8 mmol/l | <4.8 mmol2/l2 preferably < 4.2 mmol2/l2 | 2–4 × upper limit of normal range | N/A |
Summary
Serum calcium and phosphate have been associated with mortality in dialysis patients and are important in the strategy of controlling all aspects of CKD–MBD. A large number of treatment options are now available: diet, calcium content of the dialysate, phosphate binders, vitamin D, calcimimetics and parathyroidectomy. Good management is still a goal difficult to achieve and no single treatment is the answer. Increased understanding of the underlying mechanisms will hopefully improve with time and our management may change accordingly. There is still much research to be done in this area and many questions remain unanswered.
Conflict of interest statement. James Heaf: lectureship fees and travel support from Baxter, Gambro, Genzyme, Leo-Pharma, Swedish Orphan, Abbott.
References
- 1.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Beto JA, Coronado BE, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 3.Covic A, Gusbeth-Tatomir P, Goldsmith DJ. Arterial stiffness in renal patients: an update. Am J Kidney Dis. 2005;45:965–977. doi: 10.1053/j.ajkd.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Picton ML, Moore PR, Mawer EB, et al. Down-regulation of human osteoblast PTH/PTHrP receptor mRNA in end-stage renal failure. Kidney Int. 2000;58:1440–1449. doi: 10.1046/j.1523-1755.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodman WG, Ramirez JA, Belin TR, et al. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int. 1994;46:1160–1166. doi: 10.1038/ki.1994.380. [DOI] [PubMed] [Google Scholar]
- 6.Goodman WG. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial. 2004;17:209–216. doi: 10.1111/j.0894-0959.2004.17308.x. [DOI] [PubMed] [Google Scholar]
- 7.Heaf JG. Causes and consequences of adynamic bone disease. Nephron. 2001;88:97–106. doi: 10.1159/000045968. [DOI] [PubMed] [Google Scholar]
- 8.Barreto FC, Barreto DV, Moyses RM, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73:771–777. doi: 10.1038/sj.ki.5002769. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 10.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 11.London GM, Guerin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 12.Saab G, Young DO, Gincherman Y, et al. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract. 2007;105:c132–c138. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 13.Eknoyan G, Levin A, Levin NW. National Kidney Foundation: K/DOQI clinical practice guidelines: bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 14.Cuppari L, Carvalho AB, Draibe SA. Vitamin D status of chronic kidney disease patients living in a sunny country. J Ren Nutr. 2008;18:408–414. doi: 10.1053/j.jrn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 15.van Direl M, Koedam M, Buurman CJ, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 16.Gallieni M, Kamimura A, Ahmed A, et al. Kinetics of monocyte 1 alpha-hydroxylase in renal failure. Am J Physiol. 1995;268:F746–F753. doi: 10.1152/ajprenal.1995.268.4.F746. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D deficiency. New Eng J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 18.Dusso AS, Finch J, Brown A, et al. Extrarenal production of calcitriol in normal and uremic humans. J Clin Endocrinol Metab. 1991;72:157–164. doi: 10.1210/jcem-72-1-157. [DOI] [PubMed] [Google Scholar]
- 19.Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 20.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez EA, Sachdeva A, Oliver DA, et al. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24:503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 22.Zisman AL, Hristova M, Ho LT, et al. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27:36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 23.Al-Aly Z, Qazi RA, Gonzaléz EA, et al. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50:59–68. doi: 10.1053/j.ajkd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Chandra P, Binongo JN, Ziegler TR, et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract. 2008;14:10–17. doi: 10.4158/EP.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooienga L, Fried L, Scragg R, et al. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis. 2009;53:408–416. doi: 10.1053/j.ajkd.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Marsell R, Grundberg E, Krajisnik T, et al. Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol. 2008;158:125–129. doi: 10.1530/EJE-07-0534. [DOI] [PubMed] [Google Scholar]
- 27.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.London GM, Marty C, Marchais SJ, et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 30.London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 31.Schoppet M, Shroff RC, Hofbauer LC, et al. Exploring the biology of vascular calcification in chronic kidney disease: what's circulating? Kidney Int. 2008;73:384–390. doi: 10.1038/sj.ki.5002696. [DOI] [PubMed] [Google Scholar]
- 32.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Mathew S, Tustison KS, Sugatani T, et al. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewence AE, Bootman M, Roderick HL, et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–e34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 35.Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 36.London GM, Blacher J, Pannier B, et al. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 37.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 38.Qunibi W, Moustafa M, Muenz LR, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 39.Block GA, Raggi P, Bellasi A, et al. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 40.Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72:1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 41.Mehrotra R, Martin KJ, Fishbane S, et al. Higher strength lanthanum carbonate provides serum phosphorus control with a low tablet burden and is preferred by patients and physicians: a multicenter study. Clin J Am Soc Nephrol. 2008;3:1437–1445. doi: 10.2215/CJN.04741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Persy VP, Behets GJ, Bervoets AR, et al. Lanthanum: a safe phosphate binder. Semin Dial. 2006;19:195–199. doi: 10.1111/j.1525-139X.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 43.Hutchison AJ, Barnett ME, Krause R, et al. Long-term efficacy and safety profile of lanthanum carbonate: results for up to 6 years of treatment. Nephron Clin Pract. 2008;110:c15–c23. doi: 10.1159/000149239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 45.Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing haemodialysis with paracalcitol or calcitriol therapy. New Eng J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 46.Brickman AS, Coburn JW, Friedman GR, et al. Comparison of effects of 1 alpha-hydroxy-vitamin D3 and 1,25-dihydroxy-vitamin D3 in man. J Clin Invest. 1976;57:1540–1547. doi: 10.1172/JCI108424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 48.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 49.Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 50.Shroff R, Egerton M, Bridel M, et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19:1239–1246. doi: 10.1681/ASN.2007090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chonchol M, Locatelli F, Abboud HE, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of Cinacalcet HCL in participants with CKD not receiving dialysis. Am J Kidney Dis. 2009;53:197–207. doi: 10.1053/j.ajkd.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Lopez I, guilera-Tejero E, Mendoza FJ, et al. Calcimimetic R-568 decreases extraosseous calcifications in uremic rats treated with calcitriol. J Am Soc Nephrol. 2006;17:795–804. doi: 10.1681/ASN.2005040342. [DOI] [PubMed] [Google Scholar]
- 53.Costa-Hong V, Jorgetti V, Gowdak LH, et al. Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery. 2007;142:699–703. doi: 10.1016/j.surg.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 54. Renal Association clinical practice guidelines, 4th edn, Module 2, complications, 2007. Available at: www.renal.org.uk/guidelines .