Abstract
Acute lithium intoxication may cause serious neurologic and cardiac manifestations, up to the patient's death. Owing to its low molecular weight, relatively small volume of distribution close to that of total body water, and its negligible protein binding, lithium can be efficiently removed by any extracorporeal modality of renal replacement therapy (RRT). However, the shift from the intracellular to the extracellular compartment, with the inherent rebound phenomenon after the end of RRT, might limit the efficacy of the conventional, short-lasting haemodialysis. There have been no published studies up to now concerning the use of sustained low-efficiency dialysis (SLED) in lithium intoxication. This report describes a woman with a voluntary acute lithium ingestion of 40 tablets of lithium carbonate (8.12 mEq lithium each). The lithium concentration increased up to 4.18 mEq/l about 24 h after admission, notwithstanding treatment with intravenous crystalloids and gastric lavage. She developed mental status changes, oliguria, hypotension and bradycardia. We started SLED (8 h) with a blood flow of 200 ml/min and countercurrent dialysate flow of 300 ml/min. Lithium serum levels decreased by 86% during treatment, and the patient fully awoke recovering a normal mental status within the first 4 h of treatment. SLED was completed safely within the prescribed time. After the end of treatment, the rebound of lithium concentration was unremarkable. Renal function fully recovered, and the patient was transferred into a psychiatric facility 3 days after admission.
Keywords: bipolar disorder, dialysis, drug toxicity, lithium, psychotropic drugs
Introduction
Lithium is a univalent cation available as a carbonate or citrate salt. Lithium salts are being used in psychiatry since the 1940s for the prophylaxis and treatment of bipolar affective disorders and severe depression.
Even though lithium is very effective in controlling manic symptoms in up to 70% of the patients, its therapeutic index is quite narrow, with toxicity manifestations beginning to appear as soon as serum levels exceed 1.5 mEq/l [1,2]. Drug overdosage may result in serious toxicity. Acute lithium poisoning (both intentional or accidental) can be acute (i.e. in lithium-naïve patients, typically within the setting of a suicide attempt) or acute-on-chronic (i.e. in patients being treated with lithium who take an overdose). In either form the main clinical manifestations are altered mental status (from confusion and lethargy to delirium and coma), muscle hyperexcitability (from fine tremor to clonus and seizures), gastrointestinal symptoms (nausea, vomiting and diarrhoea), EKG changes (sinus node dysfunction and arrest, ST segment depression and inverted T-waves) and hypotension [3,4].
Since lithium intoxication may be life threatening, it is of crucial importance to remove the drug from the body as quickly as possible. Extracorporeal depuration techniques are considered the most efficient way for achieving such a goal, because of the drug's very low molecular weight (7 Da), the small volume of distribution close to that of total body water (central volume of distribution 0.5 l/kg with slow entry into tissues and a terminal volume of distribution of 0.7–0.9 l/kg), the negligible protein binding (<10%) and the low renal clearance rate (10–40 ml/min, half-life 14–30 h) [1,2]. Thus, RRT, usually in the form of conventional haemodialysis, is recommended for any patient on chronic lithium therapy whose serum lithium levels exceed 4 mEq/l, or when serum levels as low as 2.5–4.0 mEq/l are detected in patients showing serious neurologic, cardiac or respiratory problems, or impaired lithium renal excretion [1–4].
SLED (sustained low-efficiency dialysis) is an intermittent, prolonged RRT modality recently introduced for treating acute renal failure [5,6] and acute intoxications [7,8]. It is a highly efficient, mainly diffusive RRT modality which usually lasts 8–12 h, with blood flow and dialysis fluid rates lower than those currently used in conventional dialysis [5,6]. There is no published experience about its use in lithium intoxication. In this report we describe a case of an acute, intentional lithium overdose successfully treated with SLED.
Case report
A 47-year-old woman was evaluated at the emergency department for a lithium overdose. Four hours before admission she had deliberately ingested for suicidal purposes 40 tablets of lithium carbonate (300 mg of lithium carbonate in each tablet corresponding to 8.12 mEq of lithium) and 20 ml of a 0.25% clonazepam solution. She had started lithium treatment for a bipolar disorder 2 days before, as prescribed by her psychiatrist.
On arrival the patient was alert with psychomotor slowing. A neurological examination showed alternating bouts of agitation and lethargy, along with fine tremors and hyperreflexia, but no focal deficits. The EKG documented sinus bradycardia with 50 bpm but no ST segment or T-wave abnormalities, blood pressure was 95/50 mmHg and arterial oxygen saturation 92%. Serum creatinine was 0.8 mg/l, sodium 138 mEq/l and potassium 4.8 mEq/l; liver function tests and blood count were normal. Diuresis was <30 ml/h. Initially, serum lithium levels were 1.62 mEq/l but increased up to 2.77 mEq/l 8 h later, despite initial treatment with intravenous crystalloids and gastric lavage. The patient remained oliguric and became more confused while being affected by nausea and vomiting; she was then transferred to the renal ICU for evaluation and monitoring.
At ICU admission the patient was lethargic and complained of intense nausea; pulse was 52 bpm (sinus bradycardia), blood pressure 98/50 mmHg; during the preceding 12 h she had passed 360 ml of urine only. Body weight was 74 kg (usual BW 76 kg). Blood gas analysis showed pH 7.40, PaCO2 36 mmHg, PaO2 74 mmHg and bicarbonate 21 mEq/l; serum sodium was 137 mEq/l, potassium 4.5 mEq/l, chloride 105 mEq/l and creatinine 1.2 mg/dl.
We started SLED using a double lumen 12 F, 16 cm central venous catheter positioned in the right jugular vein, the dialysis machine AK 200S Ultra (Gambro, Italy) and the F8HPS polysulfone filter (KUf 18 ml/h/mmHg, KoA urea 848 ml/min, Fresenius, Italy) with a blood flow of 200 ml/ min and countercurrent dialysate flow of 300 ml/min [9,10]. A BW increase of 1.5 kg was programmed during the 8-h treatment. Blood samples were collected every 2 h during SLED for serum lithium level measurement; urine was also collected.
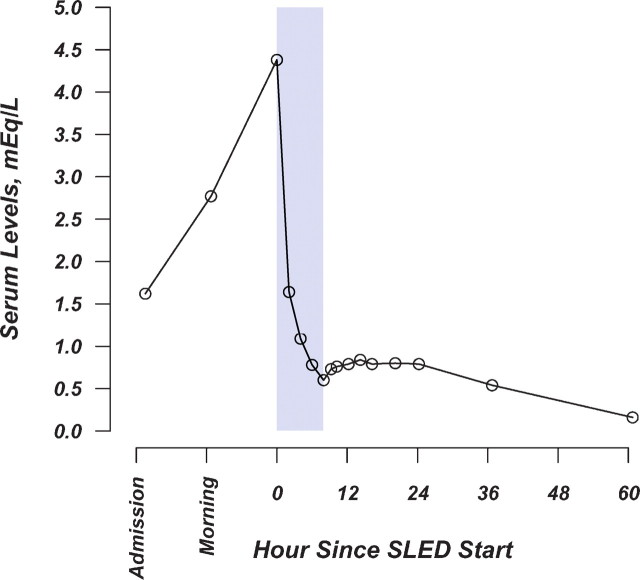
Lithium levels during and after SLED are shown in Figure 1. During SLED the urea serum level decreased by 65% and lithium serum levels by 86%. Diuresis increased sharply during treatment; nonetheless, the lithium amount recovered in the urine was 5.8 mEq only. SLED was completed safely within the prescribed time without anticoagulants. The patient fully awoke recovering a normal mental status within the first 4 h of treatment. Having completely recovered from the intoxication the patient was transferred into a psychiatric facility 3 days after admission.
Fig. 1.
Lithium levels during and after SLED. Shaded area represents SLED time.
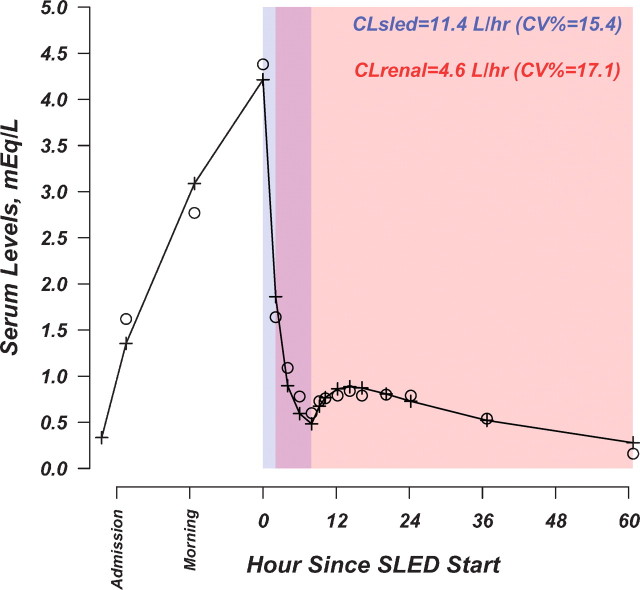
For the purpose of data analysis, differential equations describing a two-compartment open pharmacokinetic model were fit to lithium serum concentration-time data. SLED clearance and renal clearance were modelled using indicator variables. With the aim of modelling the transient oliguria at admission, the indicator variable for renal clearance was arbitrarily set to 0 at the start of the observation period and to 1 at the time when lithium decreased to levels <1.6 mEq/l (i.e. after ∼2 h of SLED). The pharmacokinetic model was fit to the data using the iterative non-linear weighted least-squares estimator in the Adapt II software package (Biomedical Simulations Resource, University of Southern California, Los Angeles, CA, USA) [11].
Observed and predicted lithium concentrations by the pharmacokinetic model are shown in Figure 2. The results of the analysis can be interpreted as follows: the progressive increase in lithium levels over the first 24 h, up to 4 mEq/l, can be accounted for by the transient oliguria detected after the admission; the high lithium clearance, achieved over the course of SLED treatment, caused a dramatic drop in lithium serum concentration. The rebound of lithium detected after the end of SLED, which can be explained by redistribution from the peripheral compartment, was only mild, being dampened by the recovery of renal function.
Fig. 2.
White circles: observed lithium concentrations. Cross symbols and connecting line: predicted lithium concentrations by the PK model. CV%: coefficient of variation (i.e. [Standard Error of The Estimate ÷ Estimate] × 100). The PK model included SLED and renal clearances as time-dependent parameters. Their relation with time is illustrated by the shaded areas, as follows: time period pertaining to SLED clearance (dark grey-shaded area); time period pertaining to renal clearance (light grey-shaded area). Renal clearance was modelled as a time-dependent discrete variable to account for the transient oliguria at admission. Estimates of the pharmacokinetic parameters were the following: renal clearance, 4.6 l/h (Coefficient of Variation%: 17.1); SLED clearance, 11.4 l/h (CV%: 15.4), central volume of distribution (Vc), 23.9 l (CV%: 12.1); peripheral volume of distribution (Vp), 19.9 l (CV%: 31.7); distributional clearance, 2 l/h (fixed); steady-state volume of distribution (Vc + Vd), 43.8 l and absorption rate constant, 0.025/h (fixed).
Discussion
This report deals with the successful treatment by SLED of an acute, voluntary lithium intoxication episode in an adult patient.
As compared with chronic or acute-on-chronic lithium intoxication, an acute overdose is thought to carry less risk, because the elimination half-life is shorter (∼12–24 h) than that observed during chronic treatment (50–60 h) [1–4]. However, even though in acute lithium intoxication clinical manifestations tend to resolve more quickly, one has to take into due account the overwhelming importance of intracellular lithium content in determining the outcome. Therefore, the indication of RRT, rather than by the mere serum drug levels, should be dictated mainly by the overall clinical status, namely, the presence of symptoms and signs of severe toxicity, the dose and timing of drug ingestion, as well as by the degree of renal function impairment [1,2,12]. Our patient had deteriorating mental status, progressively increasing serum lithium levels and oliguria over an observation period of 12 h. In such a case, the primary modality for removing lithium is RRT [1–4]. However, there is no clear evidence supporting the choice of one RRT modality over another.
Conventional haemodialysis is very effective for removing lithium; however, postdialysis drug levels often rebound [1–4,13]. Two different mechanisms may account for this rebound. First, lithium carbonate has limited aqueous solubility, which tends to delay its gastrointestinal absorption (especially when lithium is ingested in large amounts), well beyond the 4- to 8-h time period usually reported for complete absorption of non-sustained release drug preparations. Secondly, intracellular lithium slowly equilibrates with the extracellular compartment, and this efflux often continues after the end of RRT. Thus, repeated haemodialysis is often needed when serum drug values peak beyond the toxic threshold after RRT [1–4,13]. Alternatively, to overcome these inconveniences it has been suggested to employ the continuous modalities of RRT, such as continuous haemodialysis, haemofiltration or haemodiafiltration [1–4,12,13]. However, continuous RRTs do not reduce lithium levels as quickly as haemodialysis does, entail the danger of anticoagulation and usually need to be performed in an ICU environment.
SLED is an intermittent, prolonged extracorporeal RRT modality that is being increasingly used for the treatment of acute renal failure. It combines most of the advantages of either the classic intermittent treatments and the continuous modalities in terms of safety, efficiency, haemodynamic stability and costs [5,6]. Moreover, it can be performed with CRRT machines within the ICU, or even with standard haemodialysis machines and filters in the nephrology wards.
In our patient, one SLED session was efficacious in reducing the initially high serum lithium levels down to the therapeutic range during the first 2 h of treatment, and to subtherapeutic levels after 8 h of SLED. After SLED, serum lithium remained at levels <1 mEq/l even after redistribution of the cation in the body water compartments. In fact, even though a rebound occurred in the 6–12 h following the end of SLED, all values remained within the therapeutic range, the highest absolute rebound value being <0.24 mEq/l, 6 h after the end of SLED. Finally, the neurologic status significantly improved with treatment. There was no haemodynamic instability, and diuresis reappeared over the course of SLED treatment.
In conclusion, RRT still represents the cornerstone for lithium removal in selected cases of acute and acute-on-chronic intoxication, even though the choice between intermittent and continuous techniques depends on local availability and logistics, need of urgently reducing lithium levels and haemodynamic status.
SLED, on the basis of its operational characteristics combining the kinetic advantages of both classic intermittent and continuous modalities [5,6], may represent the RRT of choice. In fact, it provides an initial rapid clearance of lithium with resolution of symptoms, followed by a sustained clearance minimizing the rebound; thus, it is less likely to require treatment repetition, as it may be the case with conventional haemodialysis.
Conflict of interest statement. None declared.
References
- 1.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol. 1999;10:666–674. doi: 10.1681/ASN.V103666. [DOI] [PubMed] [Google Scholar]
- 2.Waring WS. Management of lithium toxicity. Toxicol Rev. 2006;25:221–230. doi: 10.2165/00139709-200625040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Okusa MD, Crystal LJ. Clinical manifestations and management of acute lithium intoxication. Am J Med. 1994;97:383–389. doi: 10.1016/0002-9343(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 4.Ismail N. Lithium intoxication. UpToDate 2008, Version 16.1.
- 5.Marshall MR, Golper TA, Shaver MJ, et al. Sustained low-efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int. 2001;60:777–785. doi: 10.1046/j.1523-1755.2001.060002777.x. [DOI] [PubMed] [Google Scholar]
- 6.Fliser D, Kielstein JT. Technology insight: treatment of renal failure in the intensive care unit with extended dialysis. Nature Clin Pract Nephrol. 2006;2:32–39. doi: 10.1038/ncpneph0060. [DOI] [PubMed] [Google Scholar]
- 7.Lund B, Seifert SA, Mayersohn M. Efficacy of sustained low-efficiency dialysis in the treatment of salycilate toxicity. Nephrol Dial Transplant. 2005;20:1483–1484. doi: 10.1093/ndt/gfh796. [DOI] [PubMed] [Google Scholar]
- 8.Kielstein JT, Linnenweber S, Schoepke T, et al. One for all—a multi-use dialysis system for effective treatment of severe thallium intoxication. Kidney Blood Press Res. 2004;27:197–199. doi: 10.1159/000079811. [DOI] [PubMed] [Google Scholar]
- 9.Fiaccadori E, Maggiore U, Rotelli C, et al. Removal of linezolid by conventional intermittent hemodialysis, sustained low-efficiency dialysis, or continuous venovenous hemofiltration in patients with acute renal failure. Crit Care Med. 2004;32:2437–2442. doi: 10.1097/01.ccm.0000147687.06808.92. [DOI] [PubMed] [Google Scholar]
- 10.Fiaccadori E, Maggiore U, Parenti E, et al. Sustained low-efficiency dialysis (SLED) with prostacyclin in critically ill patients with acute renal failure. Nephrol Dial Transplant. 2007;22:529–537. doi: 10.1093/ndt/gfl627. [DOI] [PubMed] [Google Scholar]
- 11.D’Argenio DZ, Schumitzky A. Adapt II Release 4, User's Guide, Los Angeles, CA, Biomedical Simulations Resource, University of Southern California, 1997.
- 12.Meyer RJ, Flynn JT, Brophy PD, et al. Hemodialysis followed by continuous hemofiltration for treatment of lithium intoxication in children. Am J Kidney Dis. 2001;37:1044–1047. doi: 10.1016/s0272-6386(05)80022-8. [DOI] [PubMed] [Google Scholar]
- 13.Goodman J, Goldfarb DS. The role of continuous renal replacement therapy in the treatment of poisoning. Sem Dial. 2006;19:402–407. doi: 10.1111/j.1525-139X.2006.00194.x. [DOI] [PubMed] [Google Scholar]