Abstract
BK virus (BKV) is a human polyomavirus. The primary infection occurs typically without specific signs or symptoms. Almost 80% of adults are seropositive. Clinically relevant infections are usually limited to individuals who are immunosuppressed. After primary infection, BKV remains latent in the kidneys and can be reactivated in the setting of immunosuppression. BKV is associated with tubulointerstitial nephritis and ureteric stenosis in renal transplant recipients. Furthermore, BKV-induced haemorrhagic cystitis (HC) is a severe complication of bone marrow transplantation (BMT) in children and adults. A combination of HC and tubulointerstitial nephritis in a patient has not been reported so far. We report on an 11-year-old boy with acute myeloid leukaemia undergoing BMT. BKV infection was reactivated during post-transplant immunosuppressive therapy causing HC associated with tubulointerstitial nephritis.
Keywords: bone marrow transplantation, haemorrhagic cystitis, interstitial nephritis, immunodeficiency, polyomavirus
Introduction
BK virus (BKV), a human polyomavirus, is a small non-enveloped DNA virus of 45 nm and seems to be very well adapted to its human host [1]. It has a circular, double-stranded DNA genome that replicates in the host nucleus [2]. BKV was first isolated in 1971 from the urine of a 39-year-old man who developed ureteric stenosis 4 months after renal transplant. The name of the virus refers to the first patient's initials, B.K. [2,3]. Primary infections occur typically without specific signs or symptoms [1]. Seventy to eighty percent of adults are seropositive [3]. BKV persists in the kidney as the principal site from where reactivation and asymptomatic shedding into the urine take place [1]. Latent BKV can be reactivated in any setting of impairment of the immune system, e.g. immunosuppression.
Haemorrhagic cystitis (HC) is a common complication in as many as 15% of patients after bone marrow transplantation (BMT). Early onset transient HC after BMT often results from the toxicity of chemotherapeutic agents [2,3]. In 1986, Arthur et al. reported that BMT patients who excrete BKV in the urine had a fourfold higher incidence of HC compared to those without BKV viruria [4]. BKV is also associated with tubulointerstitial nephritis, known as polyomavirus-associated nephropathy (PVAN), and ureteric stenosis in renal transplant recipients [2,5]. In most cases it causes allograft dysfunction and renal loss [5]. There are increasing reports of BKV causing nephropathy and cystitis in non-renal solid organ transplant recipients and other immunodeficiency diseases. This might be related to the use of more potent immunosuppressive regimens or increasing awareness of BKV as an important pathogen. However, it has been rarely described after BMT.
Due to the establishment of an accurate non-invasive screening procedure measuring polyomavirus BK-viraemia, BK-viruria and decoy cells in urine, PVAN can be diagnosed at early stages [5]. Management of PVAN is mainly based on a reduction of the immunosuppressive therapy, while the impact of anti-viral therapy is not yet clear. There is no consensus on optimal antiviral therapy to date. Some reports favour using cidofovir, a potent and highly selective inhibitor of polyomaviruses [2]. Other studies report decreased rates of BKV viruria and HC among some patients receiving ciprofloxacin. In fact, the quinolone antibiotics have been shown to inhibit BKV replication [6].
Data on the combination of haemorrhagic cystitis and tubulointerstitial nephritis are scarce, especially in the pediatric population. We therefore present a case with this combination caused by BKV in a boy with acute myeloid leukaemia undergoing BMT.
Case report
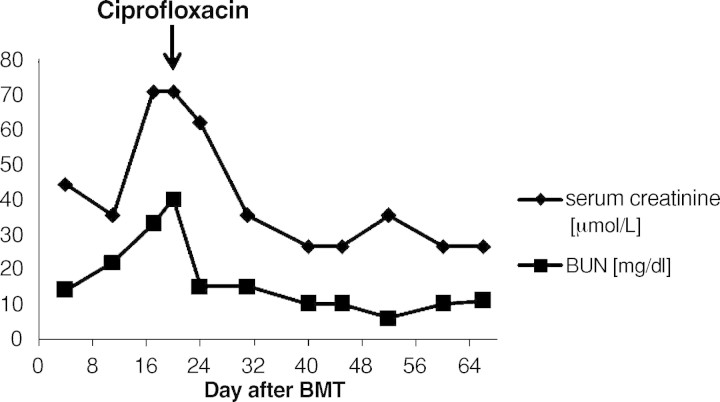
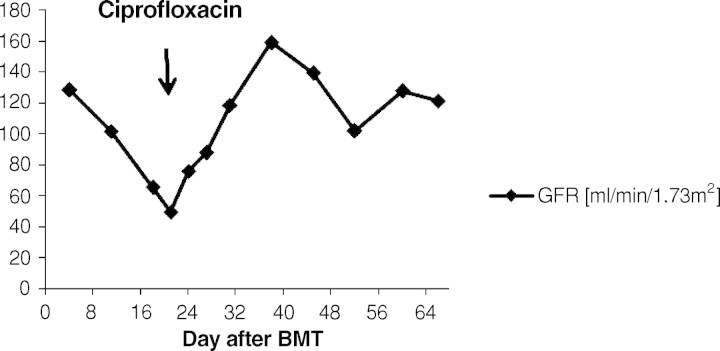
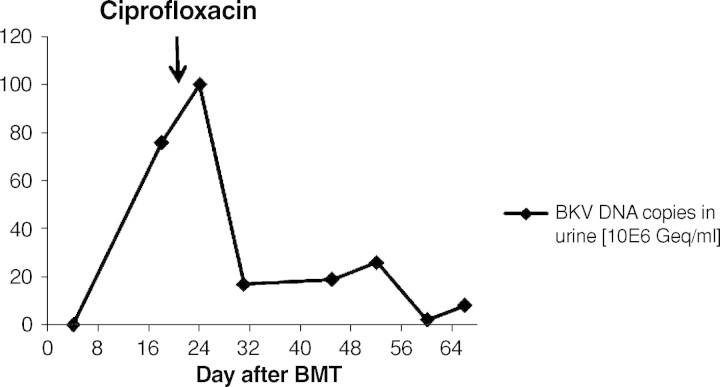
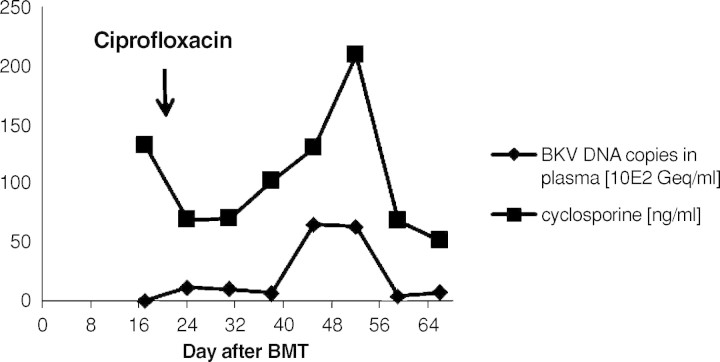
A 9-year-old boy was diagnosed with acute myeloid leukaemia (AML-M4Eo) with an inversion 16 (p13.1q22) during his vacation in France in August 2005. Initially, he was treated with induction therapy according to the French therapy study LAM. When he returned back to Germany 1 week later, treatment was continued according to the AML-BFM protocol 2004. He achieved complete remission after induction chemotherapy with idarubicin, cytarabine, mitoxantrone, etoposide phosphate and cranial radiation at Day 29. The therapy was completed uneventfully 1½ years later in February 2007. Four months later he received an isolated bone marrow relapse. Induction chemotherapy consisting of FLAG (fludarabine, cytarabine, G-CSF) resulted in a complete response in July 2007. After a second course, he was planned for matched related BMT from his HLA-identical brother. The examination before transplantation was normal. Kidney function was in the normal range. The titres indicative of past infections were positive for varicella, herpes simplex virus, rubeola, adenovirus, human herpesvirus-6 and -7 and parvovirus B19. He did not show titres against cytomegalovirus, Epstein-Barr virus and toxoplasmosis. The conditioning prior to the allogenic BMT consisted of busulfan (4 × 0.8 mg/kg/day i.v., 4 days), cyclophosphamide (2 × 60 mg/kg i.v.) and melphalan (1 × 140 mg/m2 i.v.). He was transplanted with 7 × 108 mononuclear cells/kg from the fully matched related brother. Prophylaxis against graft-versus-host disease consisted of cyclosporine 3 mg/kg/day i.v. from Day −1 (1 day before BMT). Engraftment was prompt with a WBC count (white blood cells) >1 × 109/l on Day +11, PMN count (polymorphonuclear neutrophils) >0.5 × 109/l on Day +10 and platelets >50 × 109/l on Day +10. At Day +13 the patient complained about painful macrohaematuria in the context of haemorrhagic cystitis. His serum creatinine concentration was 35 μmol/l, and the creatinine clearance was 101 ml/min/1.73 m2. Serum creatinine constantly rose within the next 4 days (Figure 1). His creatinine clearance continued to decrease, reaching a nadir of 49 ml/min/ 1.73 m2 at Day +21 consistent with progressive renal failure (Figure 4). Urine analysis results showed a gross unspecific proteinuria of 1.2 g/24 h. On microscopic examination, eumorphic and dysmorphic erythrocytes were seen. Urine cytological examination revealed transitional cells with intranuclear virus inclusions typical of polyomavirus-induced changes. Postrenal obstruction, microangiopathic nephropathy and drug toxicity were excluded as likely causes of the renal failure. Tests for antinuclear antibodies were negative. Abdomen echography showed normal kidneys and a thickening of the bladder wall with internal clots. The only pathogen isolated after extensive screening was BKV DNA in his urine (7.6 × 106 Geq/ml) (Figure 2). The plasma quantitative polymerase chain reaction (PCR) for BKV was negative at that time (Figure 3). Clinical presentation, high copy numbers of the virus in the urine and decrease of the GFR in the absence of other causes of cystitis and nephritis led to the diagnosis of ‘presumptive’ PVAN. Due to his underlying medical condition, renal biopsy, the gold standard of diagnosis BKV nephropathy, was contraindicated. Anti-BKV therapy was initiated with ciprofloxacin (2 × 10 mg/kg/day i.v.), bladder washouts by intravenous hyperhydration and reduction of cyclosporine to a trough level of 70 ng/ml was started at Day +21 after BMT. Within 3 days blood urea nitrogen (BUN) decreased and the GFR constantly rose within 7 days into the normal range (Figure 1+4). A low virus plasma load was first observed at Day + 24. At Day +52 a sudden rise in plasma virus load was detected. This was coincident with an increased cyclosporine exposure over 16 days (Figure 3), which was caused by false medication intake by the patient. Plasma virus load decreased again with lower cyclosporine exposure. Additionally, immunoglobulin replacement had been given at frequent intervals.
Fig. 1.
Course of serum creatinine and blood urea nitrogen (BUN) after BMT.
Fig. 4.
Course of the creatinine clearance. After initiation of the therapy with ciprofloxacin the GFR constantly improved within 7 days into the normal range.
Fig. 2.
Course of BKV in urine. After initiation of the therapy with ciprofloxacin at Day +21 a distinct decrease of the BKV load in urine has been observed.
Fig. 3.
Course of BKV in plasma compared to the cyclosporine levels in whole blood. There was a coincidence of an increase of the BKV in plasma and a peak of cyclosporine serum levels, which was caused by erroneously false medication intake by the patient.
The patient is doing well 9 months after BMT (Figure 5).
Fig. 5.
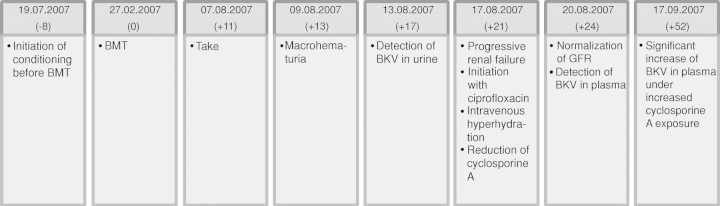
Chronological overview.
Discussion
BK virus is a ubiquitous virus, causing asymptomatic infection in over 50% of children by the age of 4 years [7]. We present a case of tubulointerstitial nephritis combined with haemorrhagic cystitis caused by possible reactivation of BKV in a boy with AML undergoing BMT.
In allogeneic bone marrow transplantat recipients, BKV is a known cause of haemorrhagic cystitis. Intravenous hydration has been shown to be effective in mild cases.
BKV nephropathy is a well-recognized complication of renal transplant patients. In renal transplant recipients, recurrent rejection episodes and high dose immunosuppressive therapy are risk factors for manifest polyomavirus kidney graft infection, which has a poor prognosis for graft survival [8]. There is no consensus on the optimal management of polyomavirus infection in immunosuppressed individuals. Nevertheless, all studies recommend renal biopsy for definitive diagnosis of BKV nephropathy. A kidney biopsy was not performed in our patient because of thrombopenia, leucopenia and immunosuppressive therapy.
Treatment of BKV nephropathy is however challenging for post-renal transplantation or during immunosuppression after BMT. Some reports support the reduction of immunosuppression combined with surveillance for allograft rejection in patients with BKV nephropathy [2,4]. Bjorang et al. describe the use of cidofovir, a potent and highly selective inhibitor of polyomavirus [9]. Nevertheless, nephrotoxicity is the major factor limiting treatment with cidofovir [8]. Other studies describe the use of ciprofloxacin, a quinolone antibiotic, which has been shown to inhibit BKV replication. Compared to cidofovir, ciprofloxacin is probably less potent in inhibiting BKV [6]. We judiciously reduced our patient's dosage of cyclosporine A, aiming at a trough level of 70 ng/ml. Because of the high nephrotoxicity of cidofovir, we administered ciprofloxacin to suppress BKV replication. In the follow-up a complete disappearance of BKV in urine and plasma was observed in our patient. Immunoglobulin replacement, which has produced favourable outcomes in renal allograft recipients with BKV infection [10], was tolerated very well.The coincidence of the increased plasma BK virus load and elevated cyclosporine A serum levels at Day 52 post-transplant suggests that immunosuppression boosts BK virus replication, which is already described elsewhere [2].
Tubular epithelial injury, which is considered as an essential cofactor of interstitial nephritis [11], could have been caused in our patient by the conditioning treatment, especially cyclophosphamide.
As reported above, primary BKV infection occurs without symptoms. Over 50% of children and 80% of adults are seropositive [3,7]. We therefore assume a reactivation of BKV in our patient. Non-invasive diagnostic tools have been evaluated to identify patients at risk, i.e. the detection of decoy cells in the urine is 100% sensitive for BKV replication. The positive predictive value of this method for BKV nephropathy, however, is <20% [1]. Nevertheless, it might be useful to screen patients under immunosuppression for BKV in urine and plasma at regular intervals. A significant increase can be seen earlier and may avoid further complications by rapid initiation of therapy.
We recommend awareness of possible BKV infection if immunosuppressed patients develop macrohaematuria and a decrease of the creatinine clearance. In these cases, creatinine clearance should be monitored closely to detect renal insufficiency early, and to avoid drug toxicity by adaptation of the dosage. A reduction of immunosuppression should be performed if possible. Ciprofloxacin might be a treatment option.
Conflict of interest statement. None declared.
References
- 1.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. doi: 10.1034/j.1600-6143.2002.020106.x. [DOI] [PubMed] [Google Scholar]
- 2.Pahari A, Rees L. BK virus-associated renal problems—clinical implications. Pediatr Nephrol. 2003;18:743–748. doi: 10.1007/s00467-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 3.Inaba H, Jones DP, Gaber LW, et al. BK virus-induced tubulointerstitial nephritis in a child with acute lymphoblastic leukemia. J Pediatr. 2007;151:215–217. doi: 10.1016/j.jpeds.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elidemir O, Chang IF, Schecter MG, et al. BK virus associated hemorrhagic cystitis in a pediatric lung transplant recipient. Pediatr Transplantat. 2007;11:807–810. doi: 10.1111/j.1399-3046.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro S, Robin M, Espérou H, et al. Polyomavirus nephropathy in the native kidneys of an unrelated cord blood transplant recipient followed by a disseminated polyomavirus infection. Transplantation. 2006;82:292–293. doi: 10.1097/01.tp.0000226172.68372.f9. [DOI] [PubMed] [Google Scholar]
- 6.Leung AY, Chan MTL, Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528–537. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 7.Kausmann JY, Somers GR, Francis DM, et al. Association of renal adenocarcinoma and BK virus nephropathy post transplantation. Pediatr Nephrol. 2004;19:459–462. doi: 10.1007/s00467-003-1407-7. [DOI] [PubMed] [Google Scholar]
- 8.Stracke S, Helmchen U, von Müller L, et al. Polyoma virus-associated interstitial nephritis in a patient with acute myeloic leukaemia and peripheral blood stem cell transplantation. Nephrol Dial Transplant. 2003;18:2431–2433. doi: 10.1093/ndt/gfg361. [DOI] [PubMed] [Google Scholar]
- 9.Bjorang O, Tveitan H, Midvedt K, et al. Treatment of polyomavirus infection with cidofovir in a renal-transplant recipient. Nephrol Dial Transplant. 2002;25:319–320. doi: 10.1093/ndt/17.11.2023. [DOI] [PubMed] [Google Scholar]
- 10.Sener A, House AA, Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81:117–120. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 11.Fishman JA. BK virus nephropathy—polyomavirus adding insult to injury. N Engl J Med. 2002;347:527–530. doi: 10.1056/NEJMe020076. [DOI] [PubMed] [Google Scholar]