Abstract
Background. Controversy exists with volume resuscitation using crystalloids or colloids. Renal dysfunction has been reported with some colloids and osmotic agents, but remains poorly defined.
Patient. We report the case of a 67-year-old male who had normal kidney function at baseline and who developed anuric ARF in relation to the administration of >10 litres of 10% pentastarch. A renal biopsy confirmed hydropic changes in tubular cells compatible with colloid-induced damage.
Conclusion. This case demonstrates that hydroxyethyl starch preparations may be associated with acute kidney injury, and one should carefully consider their use, especially in patients with pre-existing renal dysfunction. Osmotic tubular cell lesions may be long lasting and irreversible.
Keywords: acute renal failure, colloid, hydroxyethyl starch, pentastarch
Background
Volume replacement in the acutely ill and the choice between colloids versus crystalloids has generated much controversy recently. Artificial colloids such as hydroxyethyl starch (HES), gelatin and dextran have been used to obtain optimal volemia. Hydroxyethyl starches are widely used because of their favourable cost and their lack of infectious potential when compared to albumin [1]. Hydroxyethyl starches are heterogeneous molecules that are produced by hydrolysis and hydroxyethylation of amylopectin. Glucose molecules are hydroxyethylated at the C2, C3 and C6 positions to slow renal clearance. Each HES preparation is characterized by its molecular weight (70–450 kD), concentration (3, 6 and 10%), molar substitution and degree of substitution (0.4 to 0.7). HES preparations with a higher molecular weight, ratio of C2:C6 hydroxyethylation and degree of substitution have a slower metabolism and elimination. These characteristics dictate in vivo pharmacokinetics properties of HES [2]. Large HES molecules are cleaved by alpha-amylase and excreted in the urine or phagocytozed by the reticuloendothelial system, but molecules <50 kD are eliminated by glomerular filtration [3]. Traditionally, preparations of HES used in North America have been of higher molecular weights and degrees of substitution when compared to products used in Europe [1,2]. Several clinical trials, cohort studies and case reports have described renal dysfunction associated with colloids. Controversy concerns not only the safety of colloids with regards to renal function, but also the different safety profiles of the various preparations of HES.
Case report
A 67-year-old Caucasian male with known coronary artery disease, type II diabetes, hypertension, peripheral vascular disease and normal renal function was admitted with septic arthritis of the right knee. Four months before his admission, he underwent coronary artery bypass and developed a Staphylococcus aureus infection at the site of the saphenectomy. His 6-month admission was complicated due to numerous septicemias including disseminated Staphylococcus aureus infections involving the lungs, the right knee, the sternal wound and the surgical wound at the right femur. He underwent five surgical revisions and was mechanically ventilated for a prolonged time with ARDS complicating his evolution. After the fifth surgical intervention, he became haemodynamically unstable and required vasopressors for 11 days (26 to 37). The patient received broad-spectrum antibiotics during his ICU stay.
His renal function, though normal at admission, deteriorated rapidly on Day 15. This first episode of oliguric acute renal failure (ARF) was considered multifactorial and attributed to sepsis and potentially nephrotoxic medication (COX-2 inhibitor, gentamycin, ACEI). Continuous venovenous haemofiltration was started on Day 38 of admission because of uraemia, metabolic acidosis and encephalopathy and continued for 5 days. Serum creatinine stabilized between 106 and 155 μmol/L during Days 44–64 after which, for no clear reason, the patient developed anuric ARF without any response to hydration or diuretics. Throughout his intensive care unit stay, the patient received 10.75 litres of 10% pentastarch (Pentaspan, from Dupont Pharma Inc., Canada) over a 2-month period (Days 11 to 69). Intermittent haemodialysis was initiated at that point (Day 67). Renal ultrasound showed normal sized kidneys without hydronephrosis. The urinary sediment showed only two dirty brown casts and no eosinophilia. The fractional excretion of sodium was 1.2% while receiving furosemide. A renal radionuclide study showed marked bilateral hypoperfusion with a renal blood flow of 255 ml/min (normal >600 ml/min). Renal biopsy showed severe hydropic changes of the cytoplasm of tubular cells clearly suggesting pentaspan as a cause (Figures 1 and 2). The patient was discharged from our hospital after a 6-month stay. He remains on chronic haemodialysis at the present time.
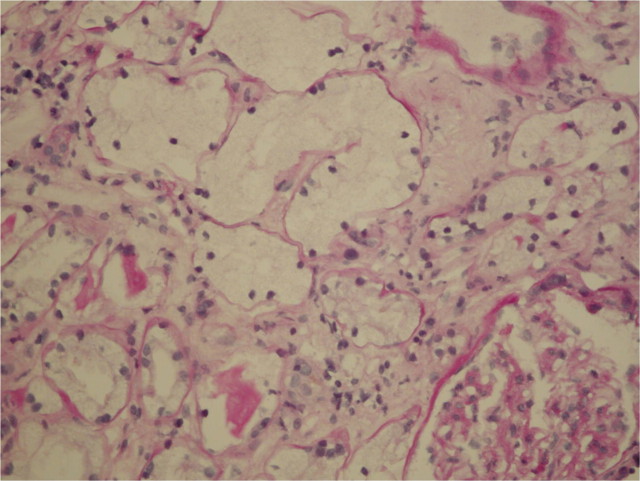
Fig. 1.
Percutaneous biopsy of the kidney, light microscopy. Periodic acid-Schiff-stained section with markedly expanded tubules. The hydropic tubular degenerescence is observed with the pale cytoplasm and flattened brush border. Few normal tubules at the lower left for comparison.
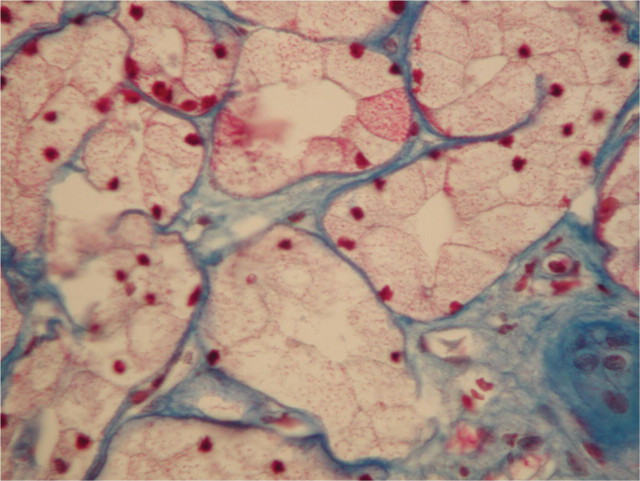
Fig. 2.
Percutaneous biopsy of the kidney, light microscopy. Trichrome de Masson-stained section showing microvacuoles in the cytoplasm of dilated tubular cells.
Discussion
Some studies showed that HES administration has led to reversible swelling in renal tubular cells by probable reabsorption of macromolecules causing tubular obstruction and medullary ischaemia [4–6]. Glomerular filtration of hyperoncotic molecules causes tubular flow stasis and obstruction of the lumen [6,7]. A lower effective glomerular filtration pressure following an increase of the plasma oncotic pressure by hyperoncotic colloids may also explain renal dysfunction induced by HES [6,8,9]. Osmotic nephrosis-like lesions (vacuolization of the proximal tubular cells) involving the proximal and distal tubules on histology and acute renal failure can be observed after infusion of HES [6,10]. Similar lesions have been described with other agents like dextran, immunoglobulins, mannitol and iodinated contrasts agents [11–15]. However, the same lesions can be seen on biopsy without associated renal failure [10].
Ten percent pentastarch has a molecular weight of 200–300 kDa and a 0.4–0.5 degree of substitution (200–300/0.4–0.5). Prescribing information suggests doses between 500 and 2000 ml/day or 28 ml/kg for a 70 kg patient. Use beyond 72 h has not been studied. The French National Institute of Health and Drug Safety recommends a maximum total dose of 80 ml/kg and administration not exceeding 4 days. Ten percent pentastarch induces an expansion of volume that persists for 18–24 h. Seventy percent of a single dose of 500 ml of 10% pentastarch will be eliminated in the urine in 24 h and 80% will be eliminated in a week. Pentaspan is contraindicated in renal insufficiency with oliguria or anuria not related to hypovolemia.
Pharmacokinetics of HES in patients with renal insufficiency are only available with the HES (130/0.4) preparation [16]. Non-anuric volunteers with mild, moderate and severe renal insufficiency received a single dose of 500 ml of HES (130/0.4). Plasma accumulation of HES (130/0.4) increased with the degree of renal insufficiency [16]. HES administration was suspected as the cause of acute renal failure (ARF) in some case reports published in recent years [17,18]. However, high doses (35.9 ml/kg) of HES 6% (200/0.5) given during total hip arthroplasty showed no differences in renal function compared to patients receiving albumin [19]. Multiple dose pharmacokinetics studies show that all HES preparations with a molar substitution of >0.4 have a decreased clearance over time (except for HES 130/0.4) [16].
In the ICU, use of albumin was compared to HES in two prospective randomized trials, and showed no difference in the creatinine and urine output between groups [20,21]. Use of gelatin was compared to HES in two prospective randomized trials, one in trauma patients [22] and another in sepsis patients [15] with conflicting results. In septic patients [15], the incidence of ARF was higher with HES while in trauma patients no difference was seen between the two groups. In these four studies [15,20–22], colloids and not crystalloids were compared to another colloid, HES.
The results of the Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, a large randomized controlled trial, were recently published and demonstrated an increased incidence of renal failure in patients receiving HES versus Ringer's lactate [23]. The study had a two-by-two factorial design which compared intensive insulin therapy with conventional insulin therapy and 10% HES (200/0.5) with Ringer's lactate and involved 537 adult patients with severe sepsis or septic shock. The trial was stopped prematurely because of an increased number of hypoglycaemic events in the intensive insulin therapy group. Patients in the HES group received a median cumulative dose of 70 ml/kg and had a significantly higher rate of acute renal failure (34.9% versus 22.8%) than those receiving Ringer's lactate. More days on renal-replacement therapy (18% versus 9%) were also required by the group receiving HES when compared to Ringer's lactate. There were no overall significant differences in mortality at 28 and 90 days; however, there was a trend towards increased mortality at 90 days in patients receiving HES.
Our patient received a cumulative dose of 130 ml/kg of 10% pentastarch over a 2-month period, including before, during and after his sepsis. The biopsy obtained clearly shows pathological evidence of renal dysfunction induced by the administration of 10% pentastarch. Recent literature supports these findings and demonstrates renal toxicity of HES with various preparations, with preparations of higher molecular weights and degrees of substitution appearing more susceptible to induce renal damage. According to our case, we may conclude that HES-induced osmotic tubular cell lesions seem long lasting, definitive and irreversible.
Conflict of interest statement. None declared.
References
- 1.Treib J, Baron J-F, Grauer MT, et al. An international view of hydroxyethyl starches. Intensive Care Med. 1999;25:258–268. doi: 10.1007/s001340050833. [DOI] [PubMed] [Google Scholar]
- 2.Davidson IJ. Renal impact of fluid management with colloids: a comparative review. Eur J Anaesthesiol. 2006;23:721–738. doi: 10.1017/S0265021506000639. [DOI] [PubMed] [Google Scholar]
- 3.Boldt J. Hydroxyethylstarch as a risk factor for acute renal failure. Is a change of clinical practice indicated? Drug Saf. 2002;25:837–846. doi: 10.2165/00002018-200225120-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kief H, Englelhart K. Reabsorptive vacuolisation der gewundenen nierenhauptstuecke (sog. Osmotische nephrose) Frankf Z Pathol. 1966;75:53–59. [PubMed] [Google Scholar]
- 5.Iana A, Schwartz D. Renal tubular cellular and molecular events in acute renal failure. Nephron. 1994;68:413–418. doi: 10.1159/000188316. [DOI] [PubMed] [Google Scholar]
- 6.Boldt J. Intravascular volume replacement therapy with synthetic colloids: is there an influence on renal function. Anesth Analg. 2003;96:376–382. doi: 10.1097/00000539-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Chinitz JL, Kim KE, Onesti G, et al. Pathophysiology and prevention of dextran-40 induced anuria. J Lab Clin Med. 1971;77:76–87. [PubMed] [Google Scholar]
- 8.Druml W, Pölzleitner D, Laggner AN, et al. Dextran-40, acute renal failure, and elevated plasma oncotic pressure. N Engl J Med. 1988;318:252–253. doi: 10.1056/NEJM198801283180413. [DOI] [PubMed] [Google Scholar]
- 9.Rozich JD, Paul RV. Acute renal failure precipitated by elevated colloid osmotic pressure. Am J Med. 1989;87:358–360. doi: 10.1016/s0002-9343(89)80171-8. [DOI] [PubMed] [Google Scholar]
- 10.Legendre C, Thervet E, Page B, et al. Hydroxyethyl starch and osmotic-nephrosis-like lesions in kidney transplantation. Lancet. 1993;342:248–249. doi: 10.1016/0140-6736(93)92345-t. [DOI] [PubMed] [Google Scholar]
- 11.DiScala VA, Mautner W, Cohen JA, et al. Tubular alterations produces by osmotic diuresis with mannitol. Ann Intern Med. 1965;63:767–775. doi: 10.7326/0003-4819-63-5-767. [DOI] [PubMed] [Google Scholar]
- 12.Diomi P, Ericsson JL, Matheson NA. Effects of dextran 40 on urine flow and composition during renal hypoperfusion in dogs with osmotic nephrosis. Ann Surg. 1970;172:813–824. doi: 10.1097/00000658-197011000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreau JF, Noel LH, Droz D. Proximal renal tubular vacuolization induced by iodinated contrast media, or so-called osmotic nephrosis. Invest Radiol. 1993;28:187–190. [PubMed] [Google Scholar]
- 14.Ahsan N, Palmer BF, Wheeler D, et al. Intravenous immunoglobulin-induced osmotic nephrosis. Arch Inter Med. 1994;154:1985–1987. doi: 10.1001/archinte.154.17.1985. [DOI] [PubMed] [Google Scholar]
- 15.Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomized study. Lancet. 2001;357:911–916. doi: 10.1016/S0140-6736(00)04211-2. [DOI] [PubMed] [Google Scholar]
- 16.Jungheinrich C, Neff TA. Pharmacokinetics of hydroxyethyl starch. Clin Pharmacokinet. 2005;44:681–699. doi: 10.2165/00003088-200544070-00002. [DOI] [PubMed] [Google Scholar]
- 17.De Labarthe A, Jacobs F, Blot F, et al. Acute renal failure secondary to hydroxyethylstarch administration in a surgical patient. Am J Med. 2001;111:417–418. doi: 10.1016/s0002-9343(01)00873-7. [DOI] [PubMed] [Google Scholar]
- 18.Dickenmann MJ, Filipovic M, Schneider MC, et al. Hydroxyethylstarch-associated transient acute renal failure after epidural anesthesia for labor analgesia and Caesarean section. Nephrol Dial Transplant. 1998;13:2706. doi: 10.1093/ndt/13.10.2706a. [DOI] [PubMed] [Google Scholar]
- 19.Vogt NH, Bothner U, Lerch G, et al. Large dose administration of 6% hydroxyethyl starch 200/0,5 for total hip arthroplasty: plasma homeostasis, hemostasis, and renal function compared to use of 5% human albumin. Anesth Analg. 1996;83:262–268. doi: 10.1097/00000539-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 20.London MJ, Ho SJ, Triedman JK, et al. A randomized clinical trial of 10% pentastarch (low molecular weight hydroxyethyl starch) versus 5% albumin for plasma volume expansion after cardiac operations. J Thorac Cardiovasc Surg. 1997;97:785–797. [PubMed] [Google Scholar]
- 21.Boldt J, Müller M, Mentges D, et al. Volume therapy in the critically ill: is there a difference? Intensive Care Med. 1998;24:28–36. doi: 10.1007/s001340050511. [DOI] [PubMed] [Google Scholar]
- 22.Allison KP, Gosling P, Jones S, et al. Randomized trial of hydroxyethyl starch versus gelatin for trauma resuscitation. J Trauma. 1999;14:1517–1520. doi: 10.1097/00005373-199912000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]