Abstract
Complement factor H auto-antibodies (CFH-ab) are a rare cause (6–10%) of atypical haemolytic uraemic syndrome (aHUS). All observations previously described were retrospective and occurred in children or teenagers. We report the first case of aHUS associated with anti-CFH antibodies in an adult patient who was successfully treated by plasma exchange, corticosteroids and rituximab.
Keywords: anti-CFH autoantibodies, atypical HUS, plasma exchange, rituximab
Background
Atypical haemolytic uraemic syndrome (aHUS) is often associated with a deficit in alternative complement pathway regulation. Mutations in genes encoding complement regulatory proteins are identified in 50% of cases [1–3]. Recently, autoantibodies targeting complement factor H (CFH-ab) have been defined as a new cause of aHUS that affects young individuals [4–6]. We report the clinical course and therapeutic management of an adult aHUS patient associated with CFH-ab discovered at initial presentation.
Case report
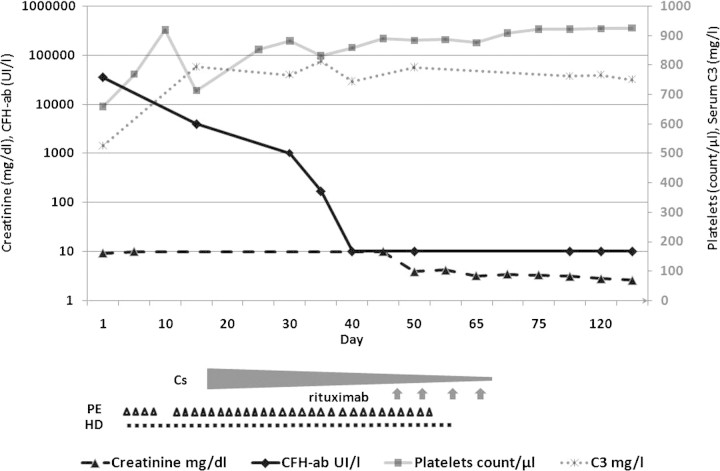
A 42-year-old man without personal or family medical history presented with acute renal failure and macroscopic haematuria. One week before, he had suffered facial oedema with spontaneous remission. He complained of abdominal pain, diarrhoea and asthenia, but did not take any medication. Physical examination revealed tachycardia (110 b.p.m.), high blood pressure (160/60 mmHg), and oliguria (450 ml/day). Laboratory evaluation showed microangiopathic haemolytic anaemia (haemoglobin 10 g/dl, unconjugated bilirubinaemia 21 mg/l, LDH 5781 UI/l, haptoglobin <0.08 g/dl, schistocytosis 5%, negative Coomb's test), thrombocytopenia (9000/μl) and elevated serum creatinine (9.2 mg/dl). Urine sample analysis revealed massive proteinuria (4 g/l) and haematuria (red blood cells >1 million/mm3). Abdominal computed tomography was normal. aHUS was diagnosed, and daily plasma exchanges (PE) with fresh frozen plasma (35 ml/kg per PE) were immediately started with intermittent haemodialysis (HD). After 4 days (Figure 1), serum platelets increased to 180 000/μl, LDH and schistocytosis decreased to 1460 UI/l and 2%, respectively. PE were stopped, but the patient remained HD dependent. After 1 week, we observed a haematological relapse (Hg 6.4 g/dl, schizocytosis 15%, LDH 4701UI/l, platelets 19 000/μl), and daily PE with fresh frozen plasma were restarted.
Fig. 1.
Clinical and therapeutic course. Top: evolution of serum creatinine (mg/dl), CFH-ab (UI/l), platelets and C3 (mg/l). Bottom: treatments with corticosteroid (Cs), plasma exchange (PE; triangle), haemodialysis (HD) and rituximab injections (arrow).
Bacteriological and immunological tests failed to demonstrate the presence of Shiga toxin-producing Escherichia coli (STEC) antibodies. ADAMTS-13 activity was within the normal range (73%), and ADAMTS-13 auto-antibodies were negative (13 UI/l). A low C3 level (525 mg/l, normal range 660–1250 mg/l) associated with a normal C4 level (280 mg/l) suggested alternative complement pathway activation. Serum FI, FH and FB levels were normal (respectively, 114%, 119% and 129 mg/l), but a high level (32 000 UI/L) of serum IgG CFH-ab was detected. Antinuclear antibodies (ANA), anti-extractable nuclear antigens (anti-ENA) and anti-double stranded DNA antibodies (anti-DNA) were negative.
Genetic analysis revealed a homozygous deletion on the complement factor H-related protein (CFHR) 1 and 3 genes and a heterozygous inversion on the IF gene (IVS12+5).
The kidney biopsy displayed a diffuse arterial and glomerular microangiopathy.
Corticosteroids were then started (1 mg/kg/day), and PE were continued three times a week. CFH-ab levels progressively decreased (Figure 1), and C3 levels returned to normal values. However, the patient remained PE dependent. Anti-CD20 immunotherapy (rituximab 375 mg/m2/week for 4 weeks) was started, and corticosteroids were progressively tapered. PE were successfully stopped after the second rituximab infusion. Renal function progressively improved, and the patient was withdrawn from HD. After 8 months follow-up, the patient remained HD-free (serum creatinine: 2.6 mg/dl) and CFH-ab was undetectable without any immunosuppressive treatment.
Discussion
The description of HUS associated with CFH-ab was first reported in 2005 by Dragon-Durey et al. Serum from 48 children with past medical history of aHUS but without mutation of CFH, IF and MCP were tested. CFH-ab was detected in three (6%) children in whom HUS had occurred at the age of 3, 9 and 10 years and was associated with diarrhoea in two cases. Relapses and extra renal involvement (liver, heart and pancreas) were frequent. One child developed end-stage renal failure. The remaining two children recovered normal renal and haematological function after PE and immunosuppressive treatment based on steroids and Azathioprine. Complement studies revealed low plasma C3 concentrations (460–574 mg/l, normal range 660–1250 mg/l). CFH levels were normal but CFH activity was decreased and negatively correlated with CFH-ab concentration. The decay-accelerating activity of the alternative pathway C3-convertase was significantly altered by CFH-ab. The authors suggested that this antibody could decrease the binding of FH to the C3bBb-convertase, thereby compromising FH inhibitor activity.
In 2008, Joszi et al. [5] published a retrospective cohort study of 147 aHUS patients. Sixteen patients (11%) aged from 5 to 17 years had CFH-ab. All of the 147 aHUS patients and 100 healthy controls were tested for plasma CFHR by Western blot analysis. Patients with CFH-ab presented a barely detectable level, or complete absence, of CFHR1 and CFHR3 in plasma. There were only eight patients with undetectable plasma CFHR1 and CFHR3 and no antibodies. These results support the hypothesis that CFHR1 and CFHR3 deletion are associated with the presence of CFH-ab. A new subform of HUS was then defined as DEAP-HUS (deficient for CFHR proteins and Factor H autoantibody positive-HUS) [6].
To our knowledge, we describe here the first case of DEAP-HUS in an adult patient. Early detection of CFH-ab led to the introduction of immunosuppressive treatment that may have contributed to partial renal recovery. We hypothesize that steroids and PE reduced CFH-ab levels. However, this strategy failed to protect the patient from subsequent relapses, and he remained PE dependent. A persistent remission could only be obtained after rituximab infusions. This suggests that a strong inhibition of the humoral immune response is required and evokes a pathological role for CFH-ab. Nevertheless, why this 42-year-old patient who presented an innate mutation of CFHR genes developed CFH-ab remains undetermined.
This observation demonstrates that DEAP-HUS can occur in adults. It points out the need for an exhaustive aetiological screening in adults presenting with an aHUS. Genotyping of complement regulatory proteins is required but the results are delayed and this technique cannot be used in routine practice. Conversely, CFH-ab can be rapidly and easily detected and might help for the early management of these patients. Thereafter, monitoring of CFH-ab levels may predict the risk of relapse but this remains to be determined.
Conflict of interest statement. None declared.
References
- 1.Loirat C, Fremeaux-Bacchi V. Hemolytic uremic syndrome recurrence after renal transplantation. Pediatr Transplant. 2008;12:619–629. doi: 10.1111/j.1399-3046.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 2.Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fremeaux-Bacchi V, Miller EC, Liszewski MK, et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dragon-Durey MA, Loirat C, Cloarec S, et al. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 5.Jozsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood. 2008;111:1512–1514. doi: 10.1182/blood-2007-09-109876. [DOI] [PubMed] [Google Scholar]
- 6.Sherka C, Jozsi M, Zipfel P, et al. Autoantibodies in haemolytic uraemic syndrome (HUS) Thromb Haemost. 2009;101:227–232. [PubMed] [Google Scholar]