Abstract
Continuous forms of renal replacement therapy (CRRT) have become established as the treatment of choice for supporting critically ill patients with acute kidney injury. Typically, these patients have activation of the coagulation cascades, peripheral mononuclear cells and platelets, but also a reduction in natural anticoagulants, and are therefore prothrombotic. For continuous modes of renal replacement therapy to be effective, in terms of both effective solute clearance and also fluid removal, the extracorporeal circuits must operate continuously. Thus, preventing clotting in the CRRT circuit is a key goal to effective patient management. As these patients may also be at increased risk of bleeding, regional anticoagulation with citrate is increasing in popularity, particularly following the introduction of commercially available CRRT machines and fluids specifically designed for citrate anticoagulation. Although regional anticoagulation with citrate provides many advantages over other systemic anticoagulants, excess citrate may lead to both metabolic complications, ranging from acidosis to alkalosis and may also potentially expose patients to electrolyte disturbances due to hyper- and hyponatraemia and hyper- and hypocalcaemia.
Keywords: anticoagulation, citrate, CRRT, haemofiltration, haemodialysis
Introduction
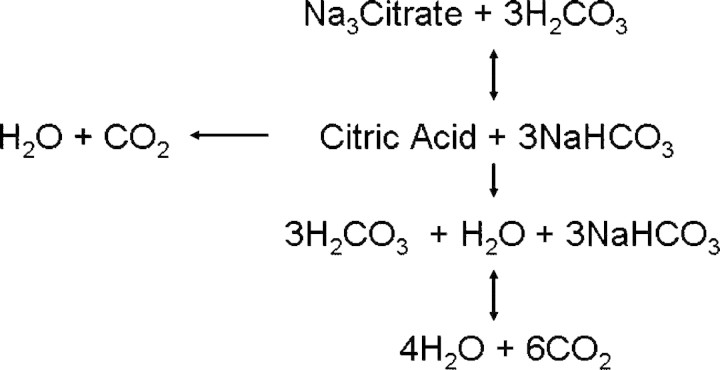
Citrate (C6H7O7) is a small negatively charged molecule with a molecular weight of 191 Daltons. In aerobic organisms, citric acid is an intermediate in the Kreb's cycle, a mitochondrial metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins to generate ATP. Intracellularly citrate is typically metabolized first to cis-aconitate, and then to d-isocitrate and α-ketoglutarate, in total liberating three carbon dioxide molecules during one full circle of the Kreb's cycle, and these can then be converted through to bicarbonate. Citrate can also be transported out of the mitochondria and into the cytoplasm, and broken down into acetyl CoA for fatty acid synthesis, whereas exogenous citrate infused as an extracorporeal anticoagulant generates sodium bicarbonate by reacting to carbonic acid (Figure 1).
Fig. 1.
Exogenous citrate can react with carbonic acid to form citric acid and sodium bicarbonate. Citric acid can then be metabolized through a number of steps to water and carbon dioxide.
For many years, citrate has been used as the anticoagulant of choice for stored blood products, typically as acid citrate dextrose (ACD), (3.22% citrate, 112.9 mmol/l citrate, 123.6 mmol/l glucose, 224.4 mmol/l sodium and 114.2 mmol/l hydrogen ions), or trisodium citrate (TCA) Na3C3H5O(COO)3, (4% TCA, 136 mmol/l citrate, 420 mmol/l sodium). Citrate chelates calcium, and at a concentration of 4–6 mmol/l with an ionized calcium of <0.2 mmol/l prevents activation of both coagulation cascades and platelets [1]. As such, citrate has been the standard anticoagulant used by haematologists and blood transfusion services for stored blood products and also as an extracorporeal anticoagulant for centrifugal platelet and leucopheresis techniques and plasma exchange. Similarly, citrate was initially used not only as an extracorporeal anticoagulant for haemodialysis more than two decades ago, initially as 4% TCA [2], but also as ACD. [3]. Citrate is essentially a regional extracorporeal anticoagulant, with a short systemic half-life of around 5 min, metabolized predominantly by mitochondria in the liver, skeletal muscle and the kidney. As citrate chelates calcium, it may help reduce some of the inflammatory reactions that occur by reducing leucocyte and monocyte activation during passage through the extracorporeal circuit [4].
CRRT and citrate anticoagulation
Continuous renal replacement therapy (CRRT) has emerged as the preferred dialysis modality for critically ill patients with acute kidney injury (AKI), particularly those with haemodynamic instability. Anticoagulation is often necessary for effective delivery of CRRT, but this requirement can also present challenges, as many critically ill patients with sepsis and/or inflammation are at both increased risk of bleeding and hypercoagulablity [5]. Without anticoagulation, the CRRT filter and circuit survival are diminished and therapy becomes less effective. Heparins are currently the most commonly used extracorporeal anticoagulants for CRRT worldwide. They are widely available, inexpensive and can be readily monitored, but have disadvantages including increased risk of haemorrhage, heparin resistance due to reduced concentrations of antithrombin III and heparin-induced thrombocytopaenia (HIT). In view of the potential side effects of heparin, alternative methods of anticoagulation have been investigated, including regional heparin/protamine, low-molecular-weight heparins, heparinoids, thrombin antagonists (hirudin and argatroban), regional citrate, prostanoids and nafamostat, with regional citrate anticoagulation (RCA) gaining wider acceptance with the development of simplified and safer protocols [6].
Citrate is infused into the blood at the start of the extracorporeal circuit and provides anticoagulation by chelating ionized calcium (iCa++). For optimal anticoagulation, the citrate infusion is adjusted to blood flow. The target extracorporeal blood citrate concentration to inhibit coagulation is 4–6 mmol/l, corresponding to a pre-filter iCa++ concentration of <0.35 mmol/l. As citrate is a small molecule, the majority of the calcium–citrate complex is freely filtered during haemofiltration or moves across the membrane by diffusion during dialysis and is lost in the ultrafiltrate or dialysate effluent. Therefore, a systemic calcium infusion is necessary to replace the calcium lost with citrate. Any calcium–citrate complex remaining then returns to the patient and is metabolized to bicarbonate by the liver, kidney and skeletal muscle. Each citrate molecule potentially yields three bicarbonate molecules, as trisodium citrate can react with carbonic acid to form sodium bicarbonate (Figure 1). Calcium released from the calcium–citrate complex helps restore normal iCa++ levels [7].
There are several potential advantages of RCA for CRRT, in that it avoids systemic anticoagulation, can act as a buffer by conversion through to bicarbonate and does not cause HIT. However, on the other hand, disadvantages include the potential for metabolic complications and the need for complex protocols [8]. The various citrate solutions reported in the literature are either customized, hospital pharmacy-formulated or commercially available. They vary in the concentration and the proportion of trisodium citrate and citric acid. As a result, equimolar dosages of citrate may differ in the amount of sodium infused and the buffer potential of the solution across different studies. The reported trisodium citrate concentrations range markedly from 0.32% up to 30%, with lower concentration solutions being used as RCA replacement solutions. Even though concentrations vary across studies, the actual citrate delivery rate to the CRRT extracorporeal circuit is less variable and typically ranges from 17 to 45 mmol/h [6].
CRRT circuit options
RCA is employed broadly across CRRT, and clinical experience with all three modalities (CVVH, CVVHD and CVVHDF) has been reported in the literature. The variations in citrate protocols are based on the composition of the citrate solution, method of citrate delivery and CRRT circuit options. Citrate solutions used for CRRT are either customized or commercially made, with the use of commercially available solutions now being more common. These commercially available solutions include anticoagulant citrate dextrose solution (ACD-A), which contains 224 mmol/l of sodium, 74.8 mmol/l of citrate and 38 mmol/l citric acid and 4% trisodium citrate solution (TCA), which contains 420 mmol/l of sodium and 136 mmol/l of citrate. Depending upon the concentration of citrate solution employed and the corresponding sodium load, compensatory hyponatraemic replacement and/or dialysate solutions with either no or reduced bicarbonate concentrations are required to prevent the development of electrolyte abnormalities [9].
Citrate is typically infused prefilter and can be delivered, either as a fixed ratio between blood and citrate infusions, or titrated based on iCa++ levels [11]. Calcium is usually administered into the systemic circulation via a separate line rather than the venous return line due to the theoretical potential of increased clotting of the access return line. However, various centres have reported success with infusing calcium in the venous return line with no apparent increase in clotting [12]. Calcium is typically given as calcium chloride that should only be given through a central line due to its sclerosing effect on peripheral veins. Alternatively, calcium gluconate can be delivered through any venous access.
Depending on the CRRT circuit design, citrate is either infused as a separate solution with the addition of a pre-filter or post-filter replacement solution, or it can be added to pre-filter replacement fluid itself [10] (Figure 1). If infused as a separate solution, the citrate is infused by a separate external IV pump through a three-way stopcock or Y-connector placed at the end of the arterial limb of the CRRT vascular access catheter [9]. As the stopcock or Y-connector is outside the CRRT circuit, net fluid removal measured by the CRRT device does not include the citrate infusion rate. Thus, nursing staff become responsible for including the amount of citrate infused when net fluid balance is calculated. Furthermore, if the CRRT device alarms for bag changes or other reasons, all pumps except the blood pump stop, resulting in the direct infusion of citrate into the patient [13].
If the citrate is infused as a pre-filter replacement fluid through the CRRT device, the citrate solution must be delivered as close to the access as possible. Otherwise, a significant amount of tubing extending from the vascular access catheter to the pre-filter replacement fluid port remains unanticoagulated, and can lead to clotting. Many CRRT devices only allow addition of the replacement solution just before blood enters the filter. The Prisma device (Gambro AB, Lund, Sweden) offers an additional pre-pump infusion setting that allows a greater portion of access line to be diluted by redirecting replacement solution close to the blood access site and before the blood pump, while the Prismaflex (Gambro AB, Lund, Sweden), which has been designed for citrate anticoagulation, has a separate fifth blood pump that allows infusion of a supplemental solution for citrate anticoagulation to be given close to the vascular access.
Continuous venovenous haemofiltration (CVVH)
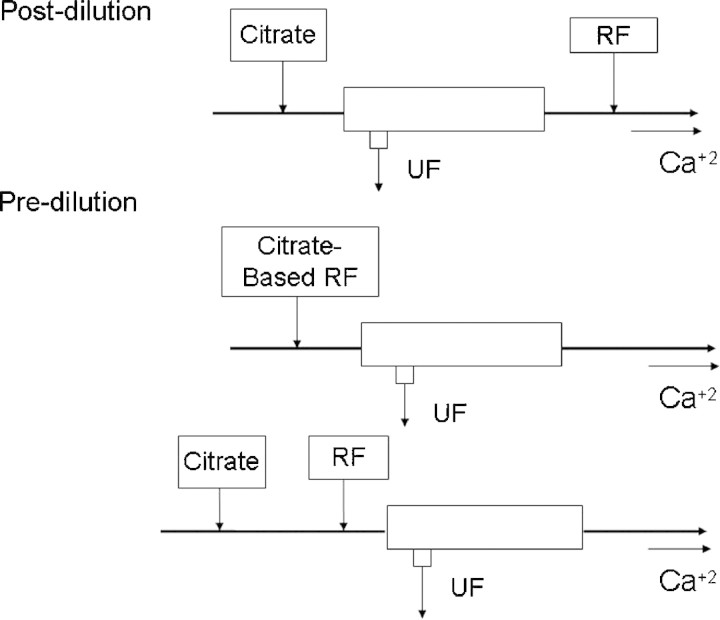
For CVVH, citrate can be infused as a separate citrate solution with addition of a pre-filter or post-filter replacement solution or added to the replacement fluid (Figure 2).
Fig. 2.
When citrate is used for continuous venovenous haemofiltration, citrate is always given pre-haemofilter, but can be administered as a separate infusion, with replacement fluids given either pre- or post-filter or as a combined citrate-replacement solution.
If citrate is infused separately, then the pre- or post-replacement fluid is usually hyponatraemic with no or minimal anionic buffer (bicarbonate or lactate) [14]. The advantage of this system is that the anticoagulation effect is not coupled to metabolic and solute control. If the citrate infusion rate and blood flow are kept constant, then the amount of citrate and bicarbonate lost in the ultrafiltrate depends upon the ultrafiltration rate [10]. If the ultrafiltration rate is increased, to increase urea and creatinine clearances, then more citrate and bicarbonate will also be lost in the ultrafiltrate [15] and the replacement solution may then need to be adjusted accordingly with increased buffer supplementation to prevent acidosis and maintain acid–base balance. This titration allows close metabolic control to be achieved. Most centres using this method use the commercially available citrate solutions. The disadvantage of this method is that the number of solutions needed is three: a citrate solution, a replacement solution and a calcium infusion. This complicates the method and increases the potential for incorrect bag placement on the CRRT device. Furthermore, the other disadvantages, as already described, is the delivery of citrate through an external IV pump.
If citrate is added to the replacement fluid, the replacement fluid is then a single solution providing both anticoagulation and convective solute clearance [10]. Therefore, this solution contains either citrate alone or a combination of citrate and bicarbonate. The advantage of this protocol is that the system is simplified by using one solution for both anticoagulation and replacement. However, since the replacement fluid rate varies with ultrafiltration flow and desired fluid removal, a fixed relation between citrate flow and blood flow is not certain. Moreover, since anticoagulation is coupled with metabolic control, the buffer cannot be titrated separately. If buffer needs are increased, then either bicarbonate has to be added to the replacement solution or administered as a separate infusion. Finally, the citrate replacement solution has to be custom-made, increasing cost, labour and allowing potential for local pharmacy compounding errors, although a dilute citrate replacement solution has recently become available in Europe known as prismocitrate (10 mmol/l trisodium citrate plus 2 mmol/l citric acid) (Gambro AB Lund, Sweden).
An alternative approach is to perform both pre- and postdilutional haemofiltration. If the citrate infusion is separate from the predilutional fluid, then this becomes a rather complex system, requiring four infusions, including the calcium re-infusion. If a combined pre-filter citrate-based predilutional solution is used, then the post-filtration option allows a greater degree of flexibility compared to simple CVVH. However, at this time, these options are not supported by the currently commercially available citrate-based solutions, and the fluid compositions required depend on the volumes exchanged, and as such require customized formulation. This approach is somewhat complex, and potential problems may occur if the predilutional fluid is connected by error to the postdilutional infusion port.
Continuous haemodialysis and haemodiafiltration CVVHD(F)
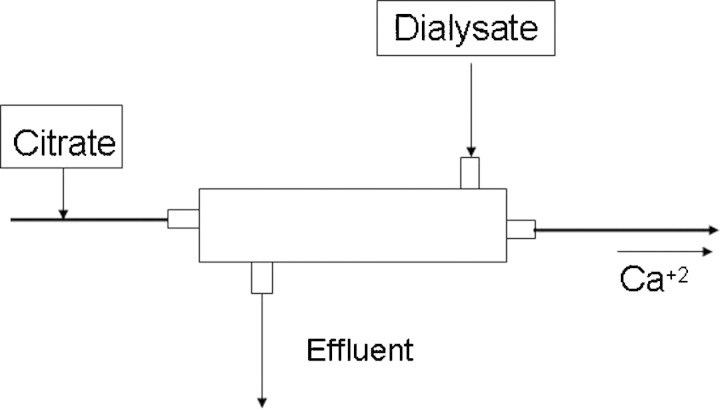
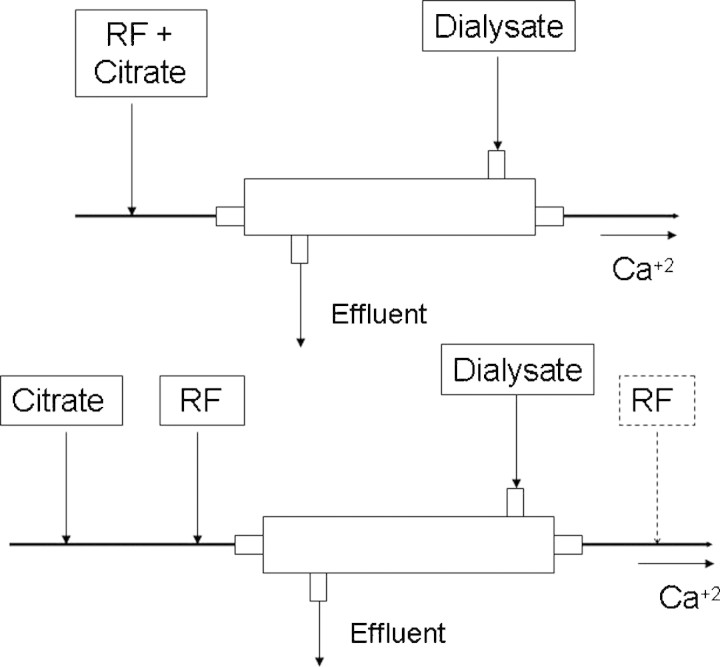
For CVVHD, citrate is infused pre-filter and typically in conjunction with a calcium-free dialysate (Figure 3). The advantage of this method is that both commercially available citrate and dialysate solutions can be used. The disadvantage is that small solute clearance is limited to diffusion and dialysate rate [16]. For CVVHDF, the citrate and replacement fluid is infused as described for CVVH with the addition of a calcium-free dialysate (Figure 4). CVVHDF has the same advantages and disadvantages as for CVVH, namely a separate citrate infusion versus a combined citrate and replacement infusion [17,18]. The only difference is that commercially available calcium-free dialysates with physiologic concentrations of electrolytes can be successfully used with both ACD-A and customized combination of citrate replacement solutions.
Fig. 3.
When citrate is used for continuous venovenous haemodialysis, then citrate is always given pre-haemofilter.
Fig. 4.
When citrate is used for continuous venovenous haemodiafiltration, citrate is always given pre-haemofilter, but can be administered as a separate infusion, with replacement fluids given either pre- or post-filter or as a combined citrate-replacement solution.
Several protocols have reported the use of a calcium-containing dialysate [18,19]. Since the calcium will chelate some of the citrate, a higher citrate infusion rate is required. Additionally, clotting in the venous air chamber has proved problematical when using calcium-containing dialysates and replacement solutions [20]. Several protocols have also described the successful use of both a citrate-based replacement solution and citrate-based dialysate for CVVHDF [21]. Using only a citrate-based dialysate is not recommended since the circuit tubing extending from the vascular access to the dialysate delivery port remains without anticoagulation and thus a potential cause for clotting.
The advantage of using a separate citrate solution, a separate replacement fluid solution and a separate dialysate solution with CVVHDF is that all commercially available solutions can be used and that anticoagulation, solute control and metabolic control can be titrated independently. However, the number of solutions required increases to four (citrate, replacement fluid, dialysate, and calcium infusion), complicating the protocol and increasing the risk of incorrect bag placement on the CRRT device by the nurse [13].
An alternative is to use customized, combined citrate replacement solutions, which contain a dilute concentration of citrate with a physiological concentration of sodium [7]. This allows for high convective rates and minimizes sodium or bicarbonate abnormalities, especially when used in conjunction with commercially available dialysates with physiologic sodium and bicarbonate concentrations. In such protocols, most metabolic derangements can be corrected with altering the rates of citrate and dialysate infusion rather than altering the composition of the solutions. Furthermore, the number of solutions required is only three. The main disadvantage of this system is that such a dilute citrate replacement solution is not universally commercially available and has to be locally made by the hospital pharmacy, the only exception being prismocitrate 10/2 (Gambro AB, Lund, Sweden) in Europe.
Comparison of circuit designs for citrate
Overall, the best circuit designs for citrate are those that are safe, simple and not labour intensive for pharmacy or nursing staff. Such protocols use either standardized or commercially available solutions and minimize the total number of CRRT solutions required.
The best solutions are those with physiological levels of sodium and bicarbonate. While haemofiltration may allow for removal of higher molecular weight solutes by convection, the addition of a dialysate to provide haemodiafiltration has a significant advantage when using citrate regional anticoagulation. Using a dialysate with a physiological solute composition provides an independent means for correction of any other electrolyte abnormalities that may occur during therapy, while maintaining a stable citrate infusion rate, especially when a combined dilute citrate solution is used for anticoagulation and replacement.
The small solute clearance achieved by CVVHDF can be increased by increasing dialysate flow, as required to treat severe metabolic acidosis or if the patient is markedly hypercatabolic and blood urea levels are not falling as expected. Increasing dialysate flow may also be effective in managing mild citrate accumulation by increasing diffusive removal of citrate. Furthermore, the use of a dialysate decreases problems with high filtration fractions or problems with decreased solute clearance from high pre-filter replacement fluid rates as seen with CVVH. Although not yet commercially available everywhere, the safest citrate solution is a dilute citrate solution with a physiological sodium concentration [7].
Monitoring of citrate anticoagulation
Due to the potential for electrolyte abnormalities, frequent monitoring of electrolytes, patient iCa++ and acid–base status is required [6]. Monitoring of the patient's blood electrolytes should be performed at least every 6 h and should include blood sodium, potassium, chloride, iCa++, magnesium and blood gas analysis along with calculation of the anion gap. At least once daily, total blood calcium concentration should be monitored to calculate the calcium ratio or calcium gap [7]. The need for monitoring anticoagulation efficacy in the circuit depends on the method of citrate delivery.
If the citrate dose is not fixed to a constant blood flow rate, then post-filter iCa++ levels should be measured at least every 6 h and the infusion of citrate titrated up or down to achieve an iCa++ target of <0.35 mmol/l. If the dose of citrate is fixed in relation to the blood flow, then less monitoring is required provided that the blood flow remains constant. If properly monitored, complications associated with regional citrate should be uncommon. It must be remembered, however, that other medical conditions, such as rhabdomyolysis, severe pancreatitis, post-tumour lysis and toxic shock syndromes may lead to hypocalcaemia, and as such this needs to be considered when reviewing the post-filter iCa++ and systemic calcium targets. Although the normal reference range for ionized calcium is 1.0–1.25 mmol/ l, the majority of proponents of regional citrate anticoagulation aim for a lower target of 0.9–1.0 mmol/l, on the basis that most critically ill patients have a lowered ionized calcium concentration, which is thought to be protective [22]. As such, lower systemic ionized calcium targets are often tolerated and tend to be treated only when patients have symptoms or signs of hypocalcaemia, or in sedated paralysed patients ECG changes such as prolongation of the QTc interval.
Metabolic complications associated with citrate anticoagulation during CRRT
While commercially available citrate solutions, such as 4% TCA and ACD-A, are commonly used for RCA in CRRT, they are not intended for anticoagulation for CRRT and have high concentrations of citrate and sodium. TCA has greater sodium content than does ACD-A (420 mmol/l versus 224 mmol/l). As a result, metabolic abnormalities can result from RCA due to the buffering capacity of citrate, the high sodium content of the citrate solutions, and the loss of calcium bound to citrate in the effluent [23]. The electrolyte abnormalities associated with citrate anticoagulation include alkalosis, acidosis, hypernatraemia and hypo- and hypercalcaemia [24]. In the setting of intensive care units and under the close supervision of a physician and nursing team trained for CRRT, these metabolic risks can be managed and prevented by altering the flow and composition of solutions delivered to the patient.
Metabolic acidosis
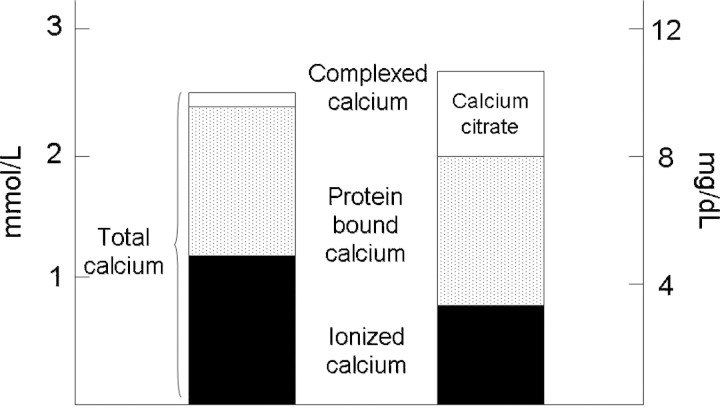
Patients with severe liver failure and lactic acidosis may have difficulty with citrate metabolism [25] and develop citrate toxicity, which is characterized by low systemic iCa++, elevated total serum calcium, metabolic acidosis and an increased anion gap [26]. The accumulation of citrate causes the systemic iCa++ concentration to fall [27], whereas the bound fraction of calcium rises. If the calcium infusion is increased to correct the low iCa++, most of the calcium is bound to citrate. A disproportional rise in total Ca occurs, while iCa++ remains low. The calcium gap (total Ca minus iCa++) or the calcium ratio (total Ca/iCa++) increases (Figure 5). Citrate toxicity is likely when the ratio of total serum calcium to ionized calcium concentration exceeds 2.5, when both total and ionized calcium are measured in mmol/l or >10 if total calcium is measured in mg/dl [28]. The correct treatment is to reduce or stop the citrate infusion, increase dialysate flow rate to increase citrate loss and also to increase the calcium infusion to correct the ionized hypocalcaemia.
Fig. 5.
If citrate cannot be metabolized, then the total serum calcium concentration appears to increase, with a corresponding fall in ionized calcium due to the increase in calcium complexed with citrate, as the calcium–citrate complex is not directly measured it is termed the ‘calcium gap’ as causing an increasing difference between total and ionized calcium.
Acidosis can occur not only due to failure to metabolize citrate through to bicarbonate, but also due to the continued losses of bicarbonate [26] and calcium citrate complexes in the dialysate effluent/filtrate.
Metabolic alkalosis
Metabolic conversion from accumulated citrate can result in an excessive alkali load [29]. The risk of developing a metabolic acidosis depends upon the amount of citrate infused. As ACD contains less citrate than 4% TCA, anticoagulation with ACD is less likely to cause metabolic alkalosis. Although citrate is negatively charged, the sieving coefficient is <1.0, as most citrate is complexed. In predilutional haemofiltration, the patients will develop a positive citrate balance, increasing with increasing volumes of citrate containing predilutional fluids. At standard slow dialysate flow rates of ∼2.0 l/h, with blood flow rates <150 ml/min, more citrate tends to be removed with dialysis-based techniques than with an equivalent predilutional haemofiltration. In routine clinical practice, the risk of developing alkalosis is dependent upon the blood flow and citrate load, with some reports of up to 50% of patients developing a metabolic alkalosis [30]. To reduce the risk of alkalosis [31], several protocols use hyperchloraemic fluids [9]. In addition, because citrate is used as a preservative for stored blood and blood products, CRRT patients in receipt of very large volumes of transfused blood may develop metabolic alkalosis while being treated with RCA. Alkalosis can be managed by either decreasing the blood flow rate, and so allowing a decrease in the citrate infusion rate into the patient, or by decreasing the infusion of citrate, or additionally by increasing citrate and bicarbonate losses in the dialysate effluent by increasing the dialysate flow, or by either reducing bicarbonate in the dialysate/replacement solutions [32] or infusing 0.9% sodium chloride as pre- or post-replacement fluid [33]. An alternative approach to reduce the risk of alkalosis has been to use less citrate, aiming for a lower pre-dialyzer/haemofilter citrate concentration of 3–4 mmol/l with a corresponding higher ionized calcium of ∼0.4 mmol/l, rather than the usual target of 4–6 mmol/l of citrate and an ionized post-filter calcium of <0.3 mmol/l, and accepting a shorter CRRT circuit life. In such cases, additional bicarbonate may be required, typically added to the dialysate.
When citrate accumulates, the proportion of ionised to bound calcium falls, as calcium binding increases, both complexed to citrate, but also to other proteins due to the pH shift, then the total ionized calcium concentration falls (Figure 5). If the fall in ionized calcium is corrected aggressively with calcium, then once citrate is metabolized and releases calcium, there is a risk of iatrogenic hypercalcaemia. As such, in cases of metabolic alkalosis due to citrate accumulation, the ionized calcium should only be supported when the patient has clinical symptoms, signs or ECG changes of hypocalcaemia.
Hypocalcaemia
Hypocalcaemia may result if citrate accumulates systemically. It may also occur by the loss of calcium bound to citrate in the effluent, or by insufficient calcium supplementation. Measurement of the calcium gap—the ratio of ionized to total calcium (Figure 5)—can help differentiate hypocalcaemia due to citrate accumulation from inadequate calcium supplementation. The ratio of ionized calcium to total calcium progressively falls, in cases of citrate accumulation and toxicity.
Calcium should preferably be infused through a separate line, and adjusted during CRRT on an ongoing basis in order to control (or would maintain be better) systemic calcium concentrations. The calcium infusion rate depends on effluent flow rate and whether or not the replacement fluid and/or dialysate contain calcium. If a calcium-containing replacement fluid and/or dialysate are used, then a separate calcium infusion may not be necessary [18]. Although the normal reference range for ionized calcium is 1.0–1.25 mmol/l, the majority of proponents of regional citrate anticoagulation aim for a lower target of 0.9–1.0 mmol/l, on the basis that most critically ill patients have a lowered ionized calcium concentration, which is thought to be protective [22]. As such, lower systemic ionized calcium targets are often tolerated and tend to be treated only when patients have symptoms or signs of hypocalcaemia, or in sedated paralysed patients, ECG changes such as prolongation of the QTc interval.
Hypernatraemia
Infusion of a concentrated TCA solution results in a very significant sodium load to the patient (420 mmol/l in a 4% TCA solution; 3060 mmol/l in a 30% TCA solution). However, hypernatraemia is an uncommon complication if using a hyponatraemic dialysate with a concentrated citrate solution or if using a citrate solution with a physiologic concentration of sodium [9].
Electrolyte balance during citrate anticoagulation
Although patients who are anticoagulated with citrate are regularly monitored and citrate and calcium infusions and dialysate and ultrafiltrate flow rates regularly adjusted according to blood test results, overall electrolyte fluxes are often not considered.
Sodium balance
Trisodium citrate is a hypernatraemic solution, and as such can lead to a positive sodium balance, particularly when used during CVVH, as the sieving coefficient of sodium is <1.0 [34], and more sodium is retained when given pre- rather than post-filter [24]. To avoid a positive sodium balance, most centres either use a pre-filter replacement solution of diluted TCA 0.4% citrate in combination with a low sodium solution e.g. 100 mmol/l, or administer a second pre-filter fluid with reduced sodium concentration pre-filter [10,14]. In theory, predilutional haemofiltration leads to a positive sodium balance, as the sieving coefficient is <1.0, and the risk of hypernatraemia is much less with dialysis modalities, and as such dialysates tend to be isonatraemic [16]. However, in routine clinical practice, sodium balance is not only dependent upon potential sodium gains from the citrate based CRRT solutions, but also sodium content of other intravenous infusions and fluids and enteral feeds, and sodium losses, including CRRT but also those from urine, drains and nasogastric suction etc. As citrate is infused pre-filter, most centres perform haemodiafiltration, with 3–4% TCA diluted by a second pre-filter fluid infusion with a hyponatraemic dialysate [9,11], or a lower citrate concentration pre-filter, such as 0.4–0.5% with an isonatraemic dialysate [7].
Calcium and magnesium balance
Citrate anticoagulation is based on calcium chelation, and as calcium citrate is lost during passage through the dialyser/haemofilter, patients become hypocalcaemic. If this is not appropriately corrected, then arrhythmias and cardiac arrest may ensue [35]. Calcium is therefore infused either systemically or into the venous return of the extracorporeal circuit to maintain normal systemic ionized calcium of 0.95–1.2 mmol/l. Since many acutely ill patients are hypoalbuminaemic and acidotic, an infusion of acidic TCA increases the relative proportion of ionized-to-bound calcium and leads to increased dialysate/filtrate calcium–citrate losses [36]. Thus, many patients treated by CRRT using citrate-based anticoagulation develop a negative calcium balance, especially when compared to standard CRRT anticoagulated with heparin, as most commercially available dialysates/replacement solutions contain high concentrations of calcium [37].
Magnesium will be similarly lost due to binding to citrate and removal of the complex during passage through the dialyser/haemofilter, and also to the effect of predilution [24]. However, unlike calcium, magnesium is added to replacement solutions and dialysates. Depending upon the volumes exchanged, higher magnesium concentrations may be required to compensate for the losses.
Clinical conditions which may potentially be associated with citrate toxicity
Citrate toxicity occurs when the amount of citrate returning to the patient can not be adequately metabolized and citrate accumulates. This may be either due to excess citrate administered by nursing or pharmacy composition error [13], circuit design and/or reduced patient metabolism. As citrate returning to the patient is complexed with calcium, this leads to a progressive increase in the total serum calcium with a reduction in the ionized calcium concentration, with the difference, termed the ‘calcium gap’ being made up of increasing calcium complexed with citrate (Figure 5) [28].
Citrate is metabolized predominantly in the mitochondria in the liver, skeletal muscle and kidney. Thus, patients with AKI treated by CRRT rely on hepatic and skeletal muscle metabolism. Thus, citrate metabolism may be compromised in patients with cardiogenic shock with reduced hepatic and muscle blood flow. In addition, patients with acute liver failure, particularly fulminant hepatic failure, cannot adequately metabolize citrate [25] and become acidotic due to continued bicarbonate and citrate losses into the dialysate/filtrate [38]. Some drugs, such as amphetamines, including 3,4-methylenedioxymethamfetamine, can lead to AKI in association with both acute liver and muscle damage. Although severe myositis or rhabdomyloysis is unlikely to cause significant citrate toxicity, provided liver function is adequate, muscle injury can cause hypocalcaemia. Most centres now monitor and regulate citrate anticoagulation by measuring systemic ionized calcium. As the ionized calcium falls in rhabdomyloysis, this can then lead to escalating systemic calcium infusion, which may later result in increased muscle damage in survivors due to calcium deposition, and a reduction in citrate infusion with increased risk of circuit clotting. Other conditions associated with hypocalcaemia include severe pancreatitis, post-tumour lysis and toxic shock.
As citrate is metabolized by mitochondria, anticoagulation with citrate may cause additional citrate toxicity in patients following accidental or deliberate poisoning with ethylene glycol and a number of mitochondrial inhibitors, including the insecticide rotenone, cyanide, antimycin, malonate, carbonyl cyanide p-[rifluoromethoxyl]-phenyl-hydrozone (FCCP), oligomycin and 2,4 dinitrophenol. In addition, patients with inherited or acquired mitochondrial cytopathies, such as mitochondrial encephalopathy with lactic acidosis and stroke episodes, and myoclonic epilepsy with ragged red fibres, may be more prone to citrate toxicity. Some of the medications taken by HIV patients have mitochondrial effects, typically more severe with stavudine than didanosine and zidovudine and lesser effects with lamivudine, emtricitabine, abacavir and tenofovir, and these may predispose to reduced citrate metabolism.
Iatrogenic errors and circuit design problems leading to acid–base imbalance
Iatrogenic problems may develop not only due to errors in fluid composition during the local manufacture and preparation of customized fluids, but also due to operational errors [13]. Whereas the typical CRRT circuit with heparin anticoagulation uses the same fluid composition for both the replacement solution and the dialysate, circuits designed for citrate anticoagulation are more complex with different fluid compositions used pre- and post-filter and for the dialysate. This can potentially lead to iatrogenic complications, if for example a pre-filter citrate replacement solution is connected to the dialysate port by accident. In addition, stopping one fluid infusion, to change to a new bag, may again lead to metabolic disturbance.
Similarly, circuit design can also lead to acid–base imbalance. Typically, this is more likely to occur with a pre-filter haemofiltration circuit, as when the amount of ultrafiltrate is increased or decreased to achieve a desired small solute (urea) clearance, correspondingly altering the citrate load.
Calcium levels have to be monitored on a regular basis, and if patients do not have indwelling arterial lines, then blood may be withdrawn from triple lumen access catheters, and in this case, samples can give erroneous calcium results, due to contamination by recirculation with returning calcium–citrate complexes in the venous return line.
The typical intensive care CRRT machine calculates fluid balance. However, when citrate is infused as a separate infusion, this volume is not assessed by the majority of the current machines, and this may result in fluid balance errors [13].
Regional citrate anticoagulation for CRRT compared to heparins
Overall, studies comparing regional citrate anticoagulation for CRRT with heparin have either reported longer or similar circuit duration and less bleeding or blood transfusion requirement with citrate [14,39,40]. There is also some evidence for reduced bio-incompatibility by decreased activation of coagulation and leucocytes with citrate [4]. In one prospective, randomized trial, median circuit lifetime was significantly longer with citrate (adjusted to maintain iCa++ in the circuit <0.3 mmol/l) compared to unfractionated heparin (UFH infusion adjusted to maintain systemic aPTT 60–80 s), 70 h versus 40 h [14]. In another randomized study, the median haemofilter survival time was 124.5 h in the citrate group compared to 38.3 h in the heparin group [39]. More recently, a larger randomized study of 215 patients reported that the efficacy and duration of CRRT circuits anticoagulated with citrate and nadroparin were similar; however, citrate was found to be safer with less bleeding and thrombocytopaenia [40]. In addition, mortality was reduced in the citrate group, with a reduction in sepsis. Whether this relates to vascular access catheter-acquired infections remains to be determined [40].
However, it must be remembered that citrate is an extracorporeal regional anticoagulant, and is therefore not a systemic anticoagulant. Thus, in cases of HIT [41], when systemic anticoagulation is required to prevent thrombosis, treatment with citrate alone is not adequate [42].
Summary
Regional citrate anticoagulation is gaining popularity for CRRT in the critically ill patient, with either similar or longer CRRT circuit life compared to standard systemic anticoagulation with unfractionated or low-molecular-weight heparins, but with reduced risk of haemorrhage and blood transfusion requirement.
The dose of citrate needs to adjusted for blood flow, to achieve a pre-haemofilter/dialyser ionized calcium target of <0.25 mmol/l, approximately corresponding to a citrate concentration of 4–6 mmol/l, for effective anticoagulation. Calcium is then re-infused to maintain a normal systemic ionized calcium concentration, and the citrate infusion adjusted according to the post-filter ionized calcium and the total systemic serum calcium.
Patients can become alkalotic due to the metabolism of an increasing citrate load returning to the patient, but also acidotic if citrate cannot be readily metabolized, or the amount of citrate infused is too low. In addition, this may be compounded by nursing and/or fluid composition errors. However, by carefully monitoring ionized and total calcium, appropriate adjustments can be made to dialysate and/or replacement fluid rates and citrate and/or calcium infusion rates, to achieve acid–base targets. Although citrate is predominantly hepatically metabolized, many patients with liver disease, even those with cirrhosis, can often adequately metabolize the citrate load.
Despite the potential pitfalls, and the initial apparent complexity of the citrate-based CRRT circuit, citrate anticoagulation is increasing in popularity due to its efficacy and safety as a regional anticoagulant for CRRT.
Conflict of interest statement. Dr Tolwani is a member of the Gambro Speakers Bureau.
References
- 1.Sakariassen KS, Ottenhof-Rovers M, Sixma JJ. Factor VIII-von Willebrand factor requires calcium for facilitation of platelet adherence. Blood. 1984;63:996–1003. [PubMed] [Google Scholar]
- 2.Pinnick RV, Wiegmann TB, Diederich DA. Regional citrate anticoagulation for haemodialysis in the patient at high risk for bleeding. N Engl J Med. 1983;308:258–261. doi: 10.1056/NEJM198302033080506. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan MJ, Pillsbury L, Sadewasser G, et al. Regional hemodialysis anticoagulation: hypertonic tri-sodium citrate or anticoagulant citrate dextrose-A. Am J Kid Dis. 1996;27:519–524. doi: 10.1016/s0272-6386(96)90162-6. [DOI] [PubMed] [Google Scholar]
- 4.Bos JC, Grooteman MP, van Houte AJ, et al. Low polymorphonuclear cell degranulation during citrate anticoagulation: a comparison between citrate and heparin dialysis. Nephrol Dial Transplant. 1997;12:1387–1393. doi: 10.1093/ndt/12.7.1387. [DOI] [PubMed] [Google Scholar]
- 5.Davenport A, Mehta S. The Acute Dialysis Quality Initiative—part VI: access and anticoagulation in CRRT. Adv Ren Replace Ther. 2002;9:273–281. doi: 10.1053/jarr.2002.35566. [DOI] [PubMed] [Google Scholar]
- 6.Davenport A. Anticoagulation for continuous renal replacement therapy. Contrib Nephrol. 2004;144:228–238. doi: 10.1159/000078891. [DOI] [PubMed] [Google Scholar]
- 7.Tolwani AJ, Prendergast MB, Speer RR, et al. A practical citrate anticoagulation continuous venovenous haemodiafiltration protocol for metabolic control and high solute clearance. Clin J Am Soc Nephrol. 2006;1:79–87. doi: 10.2215/CJN.00040505. [DOI] [PubMed] [Google Scholar]
- 8.Davenport A, Bouman C, Kirpalani A, et al. Delivery of renal replacement therapy in acute kidney injury: what are the key issues? Clin J Am Soc Nephrol. 2008;3:869–875. doi: 10.2215/CJN.04821107. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RL, McDonald BR, Aguilar MM, et al. Regional citrate anticoagulation for continuous arteriovenous haemodialysis in critically ill patients. Kidney Int. 1990;38:976–981. doi: 10.1038/ki.1990.300. [DOI] [PubMed] [Google Scholar]
- 10.Palsson R, Niles JL. Regional citrate anticoagulation in continuous venovenous hemofiltration in critically ill patients with a high risk of bleeding. Kidney Int. 1999;55:1991–1997. doi: 10.1046/j.1523-1755.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 11.Kutsogiannis DJ, Mayers I, Chin WD, et al. Regional citrate anticoagulation in continuous venovenous haemodiafiltration. Am J Kidney Dis. 2000;35:802–811. doi: 10.1016/s0272-6386(00)70248-4. [DOI] [PubMed] [Google Scholar]
- 12.Dorval M, Madore F, Courteau S, et al. A novel citrate anticoagulation regimen for continuous venovenous haemodiafiltration. Intensive Care Med. 2003;29:1186–1189. doi: 10.1007/s00134-003-1801-4. [DOI] [PubMed] [Google Scholar]
- 13.Gibney N, Cerda J, Davenport A, et al. Volume management by renal replacement therapy in acute kidney injury. Int J Artif Organs. 2008;31:145–155. doi: 10.1177/039139880803100207. [DOI] [PubMed] [Google Scholar]
- 14.Monchi M, Berghmans D, Ledoux D, et al. Citrate versus heparin for anticoagulation in continuous venovenous haemofiltration: a prospective randomized study. Intensive Care Med. 2004;30:260–265. doi: 10.1007/s00134-003-2047-x. [DOI] [PubMed] [Google Scholar]
- 15.Cassina T, Mauri R, Engeler A, et al. Continuous veno-venous hemofiltration with regional citrate anticoagulation: a four-year single-center experience. Int J Artif Organs. 2008;31:937–943. doi: 10.1177/039139880803101103. [DOI] [PubMed] [Google Scholar]
- 16.Tolwani AJ, Campbell RC, Schenk MB, et al. Simplified citrate anticoagulation for continuous renal replacement therapy. Kidney Int. 2001;60:370–374. doi: 10.1046/j.1523-1755.2001.00809.x. [DOI] [PubMed] [Google Scholar]
- 17.Swartz R, Pasko D, O’Toole J, et al. Improving the delivery of continuous renal replacement therapy using regional citrate anticoagulation. Clin Nephrol. 2004;61:134–143. doi: 10.5414/cnp61134. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell A, Daul AE, Beiderlinden M, et al. A new system for regional citrate anticoagulation in continuous venovenous haemodialysis (CVVHD) Clin Nephrol. 2003;59:106–114. doi: 10.5414/cnp59106. [DOI] [PubMed] [Google Scholar]
- 19.Gupta M, Wadhwa NK, Bukovsky R. Regional citrate anticoagulation for continuous venovenous haemodiafiltration using calcium-containing dialysate. Am J Kidney Dis. 2004;43:67–73. doi: 10.1053/j.ajkd.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Buturovic-Ponikvar J, Gubensek J, Ponikvar R. Citrate anticoagulation for postdilutional online haemodiafiltration with calcium-containing dialysate and infusate: significant clotting in the venous bubble trap. Int J Artif Organs. 2008;31:323–428. doi: 10.1177/039139880803100408. [DOI] [PubMed] [Google Scholar]
- 21.Gabutti L, Marone C, Colucci G, et al. Citrate anticoagulation in continuous venovenous haemodiafiltration: a metabolic challenge. Intensive Care Med. 2002;28:1419–1425. doi: 10.1007/s00134-002-1443-y. [DOI] [PubMed] [Google Scholar]
- 22.Forsythe RM, Wessel CB, Billiar TR, et al. Parenteral calcium for intensive care unit patients. Cochrane Database Syst Rev. 2008;4:CD006163. doi: 10.1002/14651858.CD006163.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Davenport A. Dialysate and substitution fluids for patients treated by continuous forms of renal replacement therapy. Contrib Nephrol. 2001;132:313–322. [PubMed] [Google Scholar]
- 24.Davenport A. Replacement and dialysate fluids for patients with acute renal failure treated by continuous veno-venous haemofiltration and/or haemodiafiltration. Contrib Nephrol. 2004;144:317–328. doi: 10.1159/000078899. [DOI] [PubMed] [Google Scholar]
- 25.Kramer L, Bauer E, Joukhadar C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31:2450–2455. doi: 10.1097/01.CCM.0000084871.76568.E6. [DOI] [PubMed] [Google Scholar]
- 26.Davenport A, Will EJ, Davison AM. Paradoxical increase in arterial hydrogen ion concentration in patients with hepato-renal failure given lactate based fluids. Nephrol Dial Transplant. 1990;5:432–436. doi: 10.1093/ndt/5.5.342. [DOI] [PubMed] [Google Scholar]
- 27.Nowak MA, Campbell TE. Profound hypercalcemia in continuous veno-venous haemofiltration dialysis with trisodium citrate anticoagulation and hepatic failure. Clin Chem. 1997;43:412–413. [PubMed] [Google Scholar]
- 28.Meier-Kriesche HU, Gitomer J, Kinkel K, et al. Increased total to ionised calcium ratio during continuous venovenous haemodialysis with regional citrate anticoagulation. Crit Care Med. 2001;29:748–752. doi: 10.1097/00003246-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Kelleher SP, Schulman G. Severe metabolic alkalosis complicating regional citrate anticoagulation. Am J Kidney Dis. 1987;9:235–236. doi: 10.1016/s0272-6386(87)80061-6. [DOI] [PubMed] [Google Scholar]
- 30.Morgera S, Scholle C, Voss G, et al. Metabolic complications during regional citrate anticoagulation in continuous venovenous haemodialysis: single-center experience. Nephron Clin Pract. 2004;97:c131–c136. doi: 10.1159/000079171. [DOI] [PubMed] [Google Scholar]
- 31.Davenport A, Worth DP, Will EJ. Hypochloraemic alkalosis after high-flux continuous haemofiltration and continuous arteriovenous haemofiltration with dialysis. Lancet. 1988;8586:658. doi: 10.1016/s0140-6736(88)91470-5. [DOI] [PubMed] [Google Scholar]
- 32.Cointault O, Kamar N, Bories P, et al. Regional citrate anticoagulation in continuous venovenous haemodiafiltration using commercial solutions. Nephrol Dial Transplant. 2004;19:171–178. doi: 10.1093/ndt/gfg488. [DOI] [PubMed] [Google Scholar]
- 33.Kindgen-Milles D, Amman J, Kleinekofort W, et al. Treatment of metabolic alkalosis during continuous renal replacement therapy with regional citrate anticoagulation. Int J Artif Organs. 2008;31:363–366. doi: 10.1177/039139880803100414. [DOI] [PubMed] [Google Scholar]
- 34.Davenport A. Potential adverse effects of replacing high volume haemofiltration exchanges on electrolyte balance and acid-base status using the current commercially available replacement solutions in patients with acute renal failure. Int J Artif Organs. 2008;31:3–5. doi: 10.1177/039139880803100102. [DOI] [PubMed] [Google Scholar]
- 35.Charney DI, Salmond R. Cardiac arrest after hypertonic citrate anticoagulation for chronic haemodialysis. ASAIO Trans. 1990;36:M217–M219. [PubMed] [Google Scholar]
- 36.Mehta RL, McDonald BR, Ward DM. Membrane transfer ofcitrate and calcium in regional citrate anticoagulation for continuous arteriovenous hemodialysis. J Am Soc Nephrol. 1990;1:368. [Google Scholar]
- 37.Van Der Voort PHJ, Postma SR, Kingma WP, et al. An observational study on the effects of nadroparin based and citrate based continuous venovenous haemofiltration on calcium metabolism. Blood Purif. 2007;25:267–273. doi: 10.1159/000101853. [DOI] [PubMed] [Google Scholar]
- 38.Davenport A, Aulton K, Payne RB, et al. Hyperlactataemia and increasing metabolic acidosis in hepatorenal failure treated by haemofiltration. Ren Fail. 1990;12:99–101. doi: 10.3109/08860229009087125. [DOI] [PubMed] [Google Scholar]
- 39.Kutsogiannis DJ, Gibney RT, Stollery D, et al. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int. 2005;67:2361–2367. doi: 10.1111/j.1523-1755.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 40.Oudemans-van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37:545–552. doi: 10.1097/CCM.0b013e3181953c5e. [DOI] [PubMed] [Google Scholar]
- 41.Davenport A. The management of Heparin induced thrombocytopenia during renal replacement therapy. Hemodial Int. 2001;3:81–85. doi: 10.1111/hdi.2001.5.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Davenport A. Antibodies to heparin-platelet factor 4 complex: pathogenesis, epidemiology, and management of heparin-induced thrombocytopenia in hemodialysis. Am J Kidney Dis. 2009;54:361–374. doi: 10.1053/j.ajkd.2009.03.012. [DOI] [PubMed] [Google Scholar]