Abstract
High-output cardiac failure secondary to a surgically constructed arteriovenous fistula (AVF) is a rare entity that is usually under-diagnosed in the dialysis population. We herein present a case of a 35-year-old female who was diagnosed with high-output cardiac failure secondary to an AVF and later managed with surgical division of the fistula. Risk factors associated with this entity are discussed, and preventive screening strategies are recommended.
Keywords: complication, fistula, haemodialysis, heart failure
Introduction
In view of the increase in the patient population with end-stage renal disease and the scarcity in available kidney donors, haemodialysis continues to be the primary therapy for the majority of patients with renal failure. Primary (autogenous) arteriovenous fistulas (AVFs) remain the access of choice for long-term haemodialysis patients. It is well established that AVFs carry a high risk of thrombosis and infection [1]. However, other complications including the steal syndrome and high-output cardiac failure are less encountered [1]. High-output cardiac failure usually develops in the presence of an elevated cardiac index (CI) and associated symptoms of dyspnoea, orthopnoea, paroxysmal nocturnal dyspnoea and oedema [2]. We herein present a case of high-output cardiac failure secondary to an autogenous AVF that completely resolved after surgical management of the vascular defect.
Case
The patient is a 35-year-old female, a known hypertensive, with a long-standing history of renal failure secondary to reflux nephropathy. An attempt at kidney transplantation 9 years prior to presentation failed due to graft rejection. The patient was maintained on haemodialysis therapy through a right upper extremity brachiocephalic AVF that was constructed 2 years prior to presentation, and a second attempt at renal transplantation was carried out 6 months ago. She presented to our care for investigation of bilateral lower extremity oedema. The patient complained of dyspnoea, both at rest and with exertion, paroxysmal nocturnal dyspnoea and orthopnoea. She was also unable to go about doing her daily activities. On physical examination, she was orthopnoeic and had to be supported with two pillows. Jugular venous distension was noted, and bilateral basilar crackles were evident on lung auscultation. Examination of the right upper extremity AVF revealed the presence of a severely dilated and ectatic brachiocephalic fistula with exaggerated bruit and thrill. The patient also had peripheral oedema involving both lower extremities. Compression of the AVF exaggerated the patient's dyspnoea. The diagnosis of high-output cardiac failure was entertained, and the patient was admitted to the hospital for further workup and management.
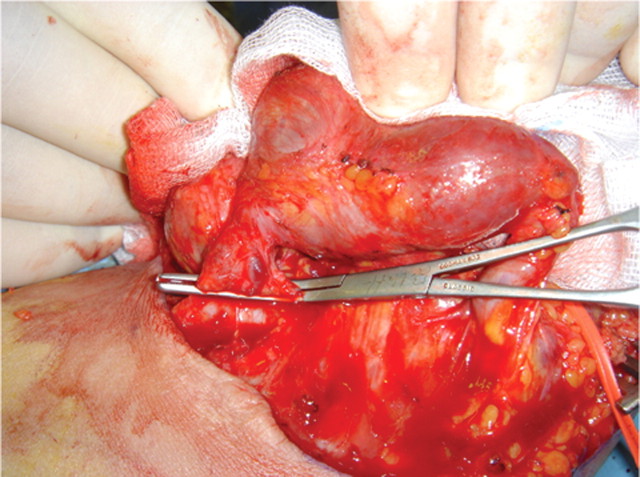
Echocardiography showed a mildly dilated left ventricle with a left ventricular diastolic dimension of 5.9 cm (normal range: 3.7–5.5 cm) and moderate dilatation of the left atrium. There was evidence of severe mitral regurgitation, and the ejection fraction was estimated at 55–59%. Her pulmonary artery pressure was estimated to be 55 mmHg. Peripheral arterial duplex scan revealed the presence of a huge AVF between the right cephalic vein and brachial artery, with an intra-fistula flow rate calculated at 3.6 l/min. After stabilizing the patient in the coronary care unit and under strict monitoring, she was transferred to the operating room. The patient underwent resection of an aneurismal right brachiocephalic AVF, and a vein patch repair of the brachial artery (Figure 1). On induction, her cardiac output was 15.91 l/min, that dropped to 8.61 l/min following a surgical repair as evident through perioperative monitoring of the pulmonary artery catheter. Post-operatively, the patient was observed in the coronary care unit for 2 days and then transferred to a regular floor before discharge. Her clinical status improved markedly with gradual disappearance of her symptoms. Her exercise tolerance increased, and the lower extremity oedema resolved with the use of diuretics. A follow-up after 1 month revealed marked improvement in the patient's status with no evidence of recurrence of symptoms.
Fig. 1.
Intraoperative image of the aneurismal arteriovenous fistula clamped prior to division.
Discussion
When compared to fistulas constructed using a prosthetic material, autogenous AVFs have superior longevity and fewer complications [1]. However, high-output cardiac failure secondary to the AVF remains a rare, yet dreadful, complication attributed to autogenous AVFs that undergo dilation of the inflow and outflow vessels resulting in large increases in flow [1]. Several factors have been associated with an increased risk of fistula-induced high-output cardiac failure, and these include location of the AVF (proximal more than distal), male sex, upper arm fistula in the same arm as a previously functioning lower arm fistula and baseline heart disease [3]. Accordingly, only the location of the AVF in our patient presents an associated risk factor.
Several studies have investigated the cut-off fistula access flow that is associated with a higher risk of high-output cardiac failure, with results ranging between 1.5 and 2.0 l/min [2,4]. Nevertheless, congestive heart failure has been described in patients with fistula flows ranging between 0.6 and 6.5 l/min [5]. Thus, it may be interpreted that cardiac failure is not only dependent on fistula flow but is also a function of myocardial reserve, as patients with adequate myocardial reserve can tolerate the increase in the haemodynamic load initiated with the presence of an AVF [5]. The haemodynamic load has been attributed to the chronic activation of the renin–angiotensin–aldosterone system and the recruitment of small increments of blood from several body stores such as the liver, spleen and splanchnic circulation [3]. The haemodynamic load may also result in an irreversible depression in the function of the left ventricle; consideration, hence, remains vital when attempting to close the fistula as early, post-operative sudden cardiac collapse and death has been previously reported [6]. Hence, patients with intrinsic cardiac disease seem to be at a higher risk of developing high-output cardiac failure in view of superimposed demands [7]. In our patient, only a previous diagnosis of hypertension was present.
In conclusion, we have described a case of high-output cardiac failure secondary to a surgically constructed autogenous AVF. Although this complication is rare, several risk factors have been described. Early recognition and management are important to avoid irreversible myocardial damage. Moreover, developing standardized screening criteria through large-scale studies would help control the morbidity and mortality associated with this complication in the already sick patient population.
Conflict of interest statement. None declared.
References
- 1.Young PR, Rohr MS, Marterre WF. High output cardiac failure secondary to a brachiocephalic arteriovenous hemodialysis fistula: two cases. Am Surg. 1998;64:239–241. [PubMed] [Google Scholar]
- 2.Basile C, Lomonte C, Vernaglione L, et al. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23:282–287. doi: 10.1093/ndt/gfm549. [DOI] [PubMed] [Google Scholar]
- 3.MacRae JM, Pandeya S, Humen DP, et al. Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis. 2004;43:e17–e22. doi: 10.1053/j.ajkd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Pandeya S, Lindsay RM. The relationship between cardiac output and access flow during hemodialysis. ASAIO J. 1999;45:135–138. doi: 10.1097/00002480-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Engelberts I, Tordoir JH, Boon ES, et al. High-output cardiac failure due to excessive shunting in a hemodialysis access fistula: an easily overlooked diagnosis. Am J Nephrol. 1995;15:323–326. doi: 10.1159/000168857. [DOI] [PubMed] [Google Scholar]
- 6.Pascual J, Martins J, Bouarich H, et al. Sudden death after arteriovenous fistula ligation in a renal transplant patient. Ann Vasc Surg. 2008;22:134–135. doi: 10.1016/j.avsg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CB, Codd JR, Graff RA, et al. Cardiac failure and upper extremity arteriovenous dialysis fistulas. Case reports and a review of the literature. Arch Intern Med. 1976;136:292–297. [PubMed] [Google Scholar]