Abstract
Sarcoidosis is a systemic disease with multiorgan involvement which can cause renal failure through several different mechanisms. Granulomatous interstitial nephritis is an important albeit less frequent cause of clinically significant renal disease. Herein, we present the case of a 46 year old woman with a history of sarcoidosis whom we evaluated for rapidly worsening kidney function and proteinuria. Renal biopsy revealed granulomatous interstitial nephritis. After therapy with adalimumab, her renal function improved with a significant reduction in proteinuria. Repeat kidney biopsy showed resolution of renal granulomata. To our knowledge, this is the first report of successful treatment of granulomatous interstitial nephritis with adalimumab.
Keywords: adalimumab, granulomatous interstitial nephritis, sarcoidosis, TNF-alpha inhibitors
Introduction
Sarcoidosis is a granulomatous multi-system disease of unknown aetiology characterized by noncaseating granulomatous inflammation with tissue destruction. TNF-alpha, which is expressed by monocytes, is critical in the development of these noncaseating granulomas. Corticosteroids are considered the mainstay of therapy. However, the use of alternative agents may be indicated if the side effects of corticosteroids are intolerable or if there is progression of disease despite adequate therapy. Many of the agents used in the therapy of sarcoidosis target, among other cytokines, TNF-alpha. These agents include nonspecific inhibitors of TNF-alpha release such as steroids, methotrexate, azathioprine, antimalarials, and phosphodiesterase inhibitors such as pentoxifylline and thalidomide. There are also nonspecific inhibitors of TNF-alpha that bind TNF-alpha preventing the initiation and perpetuation of inflammation as well as the progression of fibrosis. The family of TNF-alpha inhibitors includes three different agents. Etanercept is a soluble TNF-alpha receptor fusion protein that binds TNF-alpha. Infliximab and adalimumab are monoclonal antibodies that bind specifically to and neutralize TNF-alpha. Adalimumab blocks the interaction between the p55 and p75 cell surface TNF receptors. It also modulates the biological responses that are induced or regulated by TNF such as production of the adhesion molecules that are responsible for leukocyte migration.
Etanercept is an ineffective therapeutic agent in the treatment of sarcoidosis [1,2]. In contrast, multiple case reports and a small randomized controlled trial [3] suggest that infliximab is an effective therapy in both refractory pulmonary sarcoidosis and extrapulmonary diseases, including uveitis, neurosarcoidosis, cardiac sarcoidosis and granulomatous interstitial nephritis [4–6]. The use of adalimumab in the treatment of sarcoidosis has been limited but promising. Documented cases of the successful therapy with adalimumab include therapy of cutaneous sarcoidosis as well as the treatment of therapy-resistant multiorgan sarcoidosis [7–10]. To date, there are no documented cases of its use in the treatment of granulomatous interstitial nephritis.
Adalimumab is supplied as a sterile solution in a single use pre-filled pen or single use pre-filled syringe for subcutaneous injection. In contrast to infliximab, it can be self-administered by the patient at home. In this case report, we describe the successful treatment of a case of granulomatous interstitial nephritis using adalimumab.
Case report
A 46-year-old woman with multi-organ sarcoidosis, type 2 diabetes, subnephrotic-range proteinuria, hypertension and recurrent episodes of hypercalcaemia-induced acute kidney injury was referred for evaluation of worsening renal function and nephrotic range proteinuria. In 1987, the patient presented with an episode of iritis and was found to have hilar adenopathy. A bronchial biopsy was consistent with sarcoidosis. At the time of diagnosis, she did not have any evidence of renal involvement, but she had stage II sarcoidosis and was symptomatic from the pulmonary, ocular and neurologic points of view. Treatment with steroids resulted in multiple side effects including the successive developments of diabetes, hypertension, hyperlipidaemia and obesity. Over the next several years, multiple attempts were made to taper corticosteroid therapy, which resulted in episodes of hypercalcaemia and acute renal failure. Each episode almost completely resolved with the administration of fluids and resumption of steroids with a new baseline creatinine of 141.4 μmol/L (1.6 mg/dL). The administration of methotrexate as a steroid-sparing agent was not only ineffective but also poorly tolerated due to gastrointestinal side effects. In 2002, her proteinuria measured ∼2.6 g/24 h and her serum creatinine ranged between 159.2 μmol/L and 176.8 μmol/L (1.8 and 2.0 mg/dL). The diagnosis of multiple myeloma was excluded with a negative urine and serum electrophoresis. In 2007, she was referred to nephrology for evaluation of elevated creatinine and proteinuria in the setting of a corrected calcium level of 2.46 mmol/L (9.84 mg/dL) with an albumin of 36 g/L. At the time of evaluation, her serum creatinine was 212 μmol/L (2.4 mg/dL) and she had ∼10 g of protein excretion per day. She had poorly controlled diabetes and was on maximal dosages of an angiotensin receptor blocker but was unable to tolerate an angiotensin converting enzyme inhibitor due to persistent cough. We performed a renal biopsy due to the rapid decline in GFR in a few months and the large increase in proteinuria from baseline. The biopsy consisted of at least seven glomeruli revealing granulomatous interstitial nephritis consistent with renal sarcoidosis with moderate-to-severe chronic tubulointerstitial disease, multiple granulomas along with hypertensive vasculopathy and diabetic glomerulosclerosis with diffuse and nodular lesions (Figure 1A and B). The biopsy was not suggestive of glomerular involvement from sarcoidosis. High-dose steroids were felt to be an inappropriate therapeutic option in the setting of her poor glycaemic control and underlying diabetic nephropathy. Therefore, we chose to initiate a TNF-alpha inhibitor as an alternative therapy. She was evaluated for risk factors for tuberculosis and was found to have a negative tuberculin skin test prior to initiation of therapy. She was initiated on HumiraTM 40 mg/0.8 cc weekly for an arbitrary duration of 6 months after which we performed a follow-up renal biopsy to assess disease resolution. At the initiation of therapy, her serum creatinine was 345 μmol/L (3.9 mg/dL) and she had ∼10 g of protein excretion/24 h. After 6 months of therapy her serum creatinine improved
Fig. 1.
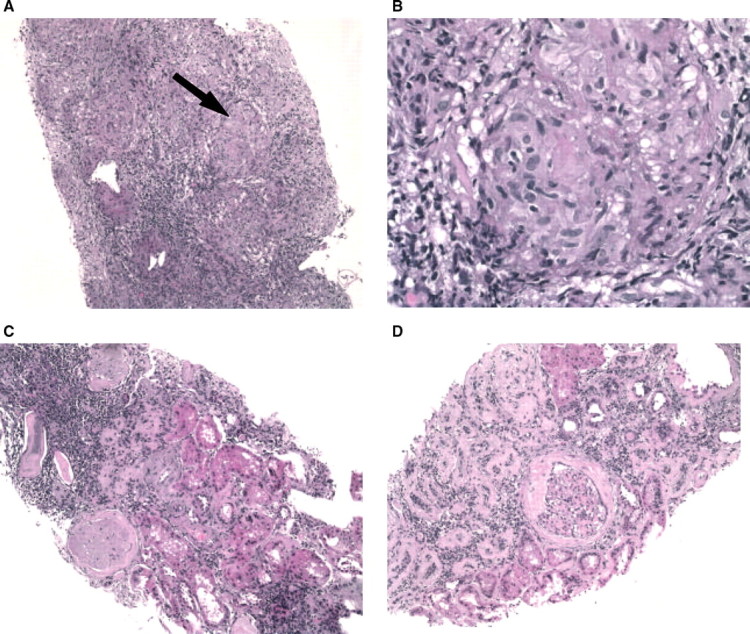
(A) Pre-treatment granulomatous interstitial nephritis (H & E, low power), arrow demonstrating granuloma. (B) Pre-treatment granulomatous interstitial nephritis granuloma (H & E, high power). (C and D) Post-treatment with adalimumab showing an intact glomerulus with diabetic changes, marked tubular atrophy, minimal interstitial inflammation and complete disappearance of granulomas (H & E, low power).
to 159 μmol/L (1.8 mg/dL) and her protein excretion declined to ∼3.5 g in 24 h (Figure 2). A repeat biopsy sampled ∼17 glomeruli and revealed stable diabetic glomerulosclerosis, moderate chronic tubulointerstitial inflammation with complete resolution of interstitial epitheliod granulomas (Figure 1C and D). This patient tolerated this therapy for 6 months with no adverse events reported.
Fig. 2.
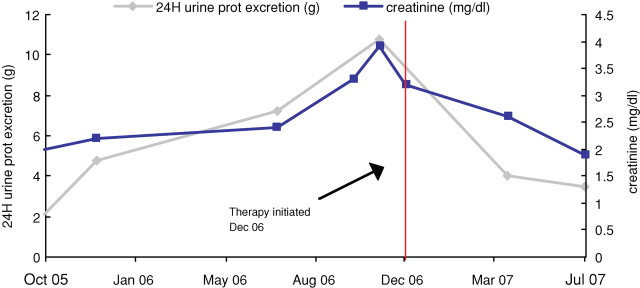
Serum creatinine and daily protein excretion in a female with granulomatous interstitial nephritis who was treated with adalimumab weekly for 6 months.
Discussion
We were able to successfully reverse granulomatous disease both functionally and histologically, by using adalimumab as an alternative agent. Although the biopsy sampling error cannot be completely excluded, the combination of clinical improvement and complete lack of granulomatous features on follow-up biopsy strongly suggest that immunomodulation by adalimumab induced resolution of sarcoid nephropathy. We sampled an adequate number of glomeruli on follow-up biopsy to fully assess the disappearance of the granulomas. Additionally, through the use of this alternative agent, we were able to avoid exacerbation of her hypertensive and diabetic disease. At initiation of therapy, we believed that clinically this patient had advanced diabetic nephropathy with proteinuria in addition to granulomatous interstitial nephritis that accounted for her acute and rapid rise in serum creatinine. Although granulomatous interstitial nephritis does not typically cause nephrotic-range proteinuria, we believe that in this case the favourable results of adalimumab treatment on proteinuria were linked to the vast improvement of the tubulointerstitial disease that is also commonly seen in diabetic nephropathy. It is known that ∼4–5 g of albumin undergo glomerular filtration each day. This is counteracted by significant protein reabsorption in the proximal tubule. Severe tubulointerstitial disease can increase proteinuria by impairing this reabsorption [11]. We believe that adalimumab improved this patient's renal function and led to a reduction in proteinuria after 6 months of therapy by alleviating the interstitial nephritis in this patient.
While selecting a medication regimen, we took into consideration that the different TNF antagonists can be associated with varying medical costs and can impact quality of life. Adalimumab is administered subcutaneously, whereas infliximab is administered intravenously. From an economic standpoint, infliximab administration requires more health care resources with requirements for infusion suites and equipment as well as medical and nursing supervision [12]. A recent paper by Walsh et al. found that the impact of switching from infliximab to adalimumab therapy in patients with rheumatoid arthritis has the potential for significant economic benefit to both the individual and to society [13]. Schwartzman et al. found that the route of administration of TNF antagonist therapy can lead to dissatisfaction with treatment and can negatively affect quality of life and lead to noncompliance with therapy, ultimately affecting long-term therapeutic outcomes [14].
Adalimumab, like infliximab, is not without systemic side effects although most are relatively minor. Among those causing the most concern is the risk of reactivation of tuberculosis. Moreover, monoclonal TNF-alpha inhibitors can cause false negative tuberculin results. Because these medications are immune modulators, patients are also at increased risk for developing infections. Finally, although no causal relationship has been established, their use has been associated with an increased incidence of lymphomas.
Differences in the response profiles of etanercept and infliximab in the treatment of granulomatous diseases like sarcoid have been conjectured to be related mainly to the differences in binding characteristics, structure and pharmokinetic profiles [15]. The shared mechanism of action of infliximab and adalimumab may explain the positive response in our case. An open label randomized controlled trial evaluating its role in the treatment of steroid resistant sarcoid disease is currently in progress, and we await its results with interest and optimism.
Conflict of interest statement. None declared.
References
- 1.Ulz JP, Limper AH, Kalra S, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124:177. doi: 10.1378/chest.124.1.177. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Lower EE, Bradley DA, et al. Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest. 2005;128:1062. doi: 10.1378/chest.128.2.1062. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 4.Kobylecki C. Refractory neurosarcoidosis responsive to infliximab. Pract Neurol. 2007;2:112–115. [PubMed] [Google Scholar]
- 5.Uthman I, Touma Z, Khoury M. Cardiac sarcoidosis responding to monotherapy with infliximab. Clin Rheumatol. 2007;11:2001–2003. doi: 10.1007/s10067-007-0614-1. [DOI] [PubMed] [Google Scholar]
- 6.Sobrin L. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125:895–900. doi: 10.1001/archopht.125.7.895. [DOI] [PubMed] [Google Scholar]
- 7.Thumfart J. Isolated sarcoid granulomatous interstitial nephritis responding to infliximab therapy. Am J Kidney Dis. 2005;45:411–414. doi: 10.1053/j.ajkd.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Callagas-Rubio JL. Treatment of therapy-resistant sarcoidosis with adalimumab. Clin Rheumatol. 2006;4:596–597. doi: 10.1007/s10067-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 9.Phillips MA. Ulcerative cutaneous sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917. doi: 10.1016/j.jaad.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Heffernan MP. Adalimumab for treatment of cutaneous sarcoidosis. Arch Dermatol. 2006;142:17–19. doi: 10.1001/archderm.142.1.17. [DOI] [PubMed] [Google Scholar]
- 11.Jefferson JA, Shankland SJ, Pickerel RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 12.Nuijten MJ, Engelfriet P, Duijin K, et al. A cost–cost study comparing etanercept with infliximab in rheumatoid arthritis. Pharmocoeconomics. 2001;19(10):1051–1064. doi: 10.2165/00019053-200119100-00006. [DOI] [PubMed] [Google Scholar]
- 13.Walsh C, Minnock P, Slattery C. Quality of life and economic impact of switching from established infliximab therapy to adalimumab in patients with rheumatoid arthritis. Rheumatology. 2007;46:1148–1152. doi: 10.1093/rheumatology/kem074. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzman S, Morgan GJ., Jr Does route of administration affect outcome of TNF antagonist therapy? Arthritis Res Ther. 2005;6(Suppl 2):S19–S23. doi: 10.1186/ar996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haraoui B. Differentiating the efficacy of the tumor necrosis factor inhibitors. Semin Arthritis Rheum. 2005;34(Suppl 1):7–11. doi: 10.1016/j.semarthrit.2005.01.003. [DOI] [PubMed] [Google Scholar]


