Abstract
Acute renal infarction is a serious medical emergency. The diagnosis is often delayed or missed as it is not common. Hence, the exact incidence of acute renal infarction is not known. Failure to consider renal infarction in the initial differential diagnosis results in a delay in diagnosis and treatment, which in turn leads to permanent loss of renal function. We present two cases of acute kidney infarction that were initially treated as renal colic. In addition, we present a third case when a kidney was saved with reperfusion therapy.
Keywords: renal infarction, renal thromboembolism, renal thrombosis
Introduction
Acute renal infarction due to renal artery thrombosis or thromboembolism is a serious medical emergency. The diagnosis is often missed and hence the exact incidence of acute renal infarction is not known. In patients with pre-existing atrial fibrillation, the incidence of renal thromboembolism is said to be ∼2% [1]. Renal infarction typically presents with acute loin pain and associated abdominal or loin tenderness. Frank or microscopic haematuria can be found in almost all patients [2]. The clinical presentation is often similar to that of renal colic due to renal calculi. Failure to consider renal infarction in the initial differential diagnosis results in delay in diagnosis and treatment, which in turn leads to permanent loss of renal function. We present two cases of acute kidney infarction that were initially managed as renal colic, and a third case when a patient's kidney was saved with reperfusion therapy.
Case A
A 41-year-old man was admitted to the hospital with acute left loin pain. He had a history of essential hypertension treated with amlodipine and atenolol. He was an ex-smoker, having a 10 pack year smoking history. His creatinine on admission was 120 μmol/L (eGFR of 54.2 mL/min/ 1.73 m2). His renal ultrasound was normal and his intravenous urogram (IVU) showed a non-excreting left kidney. A non-contrast CT scan was done which revealed some scarring of the right kidney but no renal or ureteric calculi could be demonstrated. His creatinine rose to 241 μmol/L 2 days after admission, and a JJ stent was inserted into the left ureter with a presumed diagnosis of ureteric obstruction due to calculi. He was eventually referred to the nephrologists in outpatients.
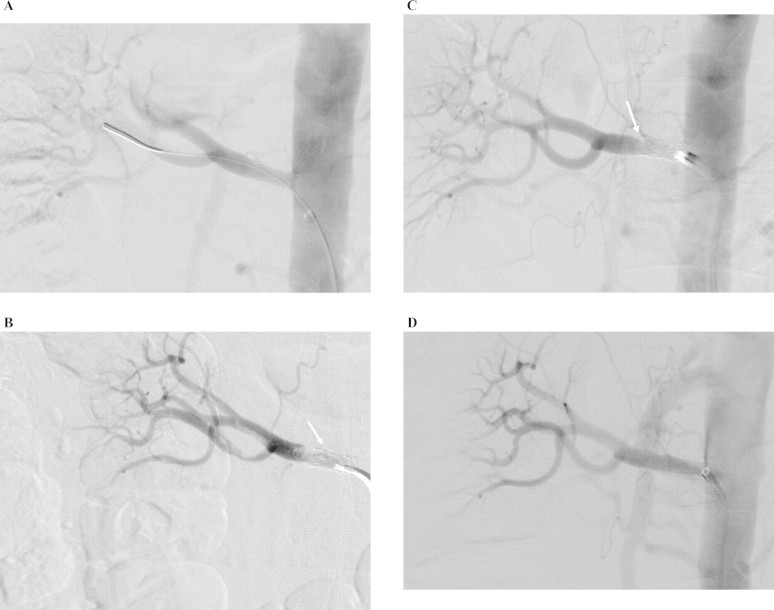
An outpatient dimercaptosuccinic acid scan (DMSA) was done 4 weeks after discharge. It showed minimal uptake within the left kidney and his right kidney contributed to almost the entirety of renal function. A subsequent CT renal angiogram showed complete occlusion of the left renal artery. He then proceeded to have a formal renal angiogram that confirmed post-osteal occlusion of the left renal artery. The main right renal artery is normally proximal and has a significant web-like stenosis in its distal portion just proximal to the polar bifurcation (Figure 1A). There was also some irregularity of the main upper pole branch more distally with slight beading that was consistent with fibromuscular dysplasia. The web-like stenosis in the distal main stem of the right renal artery was then dilated with a 6-mm balloon which produced an improved lumen (Figure 1B). The patient has been well since the procedure with a stable creatinine of ∼150 μmol/L.
Fig. 1.
Digital subtraction images of renal angiogram showing: (A) post-osteal occlusion of the left renal artery (white solid arrow). Web-like stenosis in the distal portion of the right renal artery just proximal to the polar bifurcation (white dashed arrow). (B) Improved lumen of right renal artery stenosis post-angioplasty (white dashed arrow).
Case B
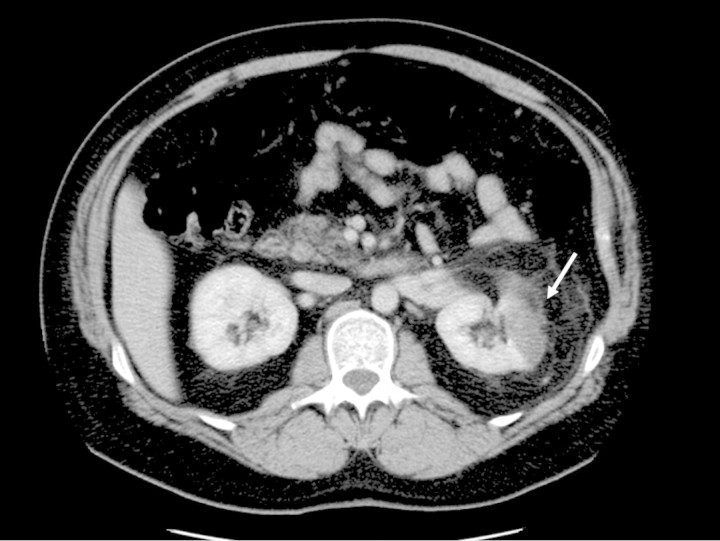
A 45-year-old man was admitted with acute left loin pain associated with vomiting. On examination, he was apyrexial and there was tenderness in the left loin. Dipstick urine analysis revealed 1+ protein only. Initial investigations including renal function, calcium and intravenous urogram (IVU) were normal. The patient was discharged the following morning with a presumed diagnosis of renal colic. However, his loin pain persisted and he was readmitted 2 days later. Repeat dipstick urine analysis showed 3+ blood and 1+ protein. A CT urogram was performed that revealed a focal segmental infarct in the left kidney (Figure 2). A subsequent CT renal angiogram showed no obvious stenosis or thrombosis in either of the renal arteries and confirmed the presence of renal infarct in the left kidney. His ECG showed that he was in sinus rhythm. His echocardiogram was normal apart from mild concentric left ventricular hypertrophy and his thrombophilia screen was normal.
Fig. 2.
CT urogram showing a segmental infract in the left kidney (white arrow).
Case C
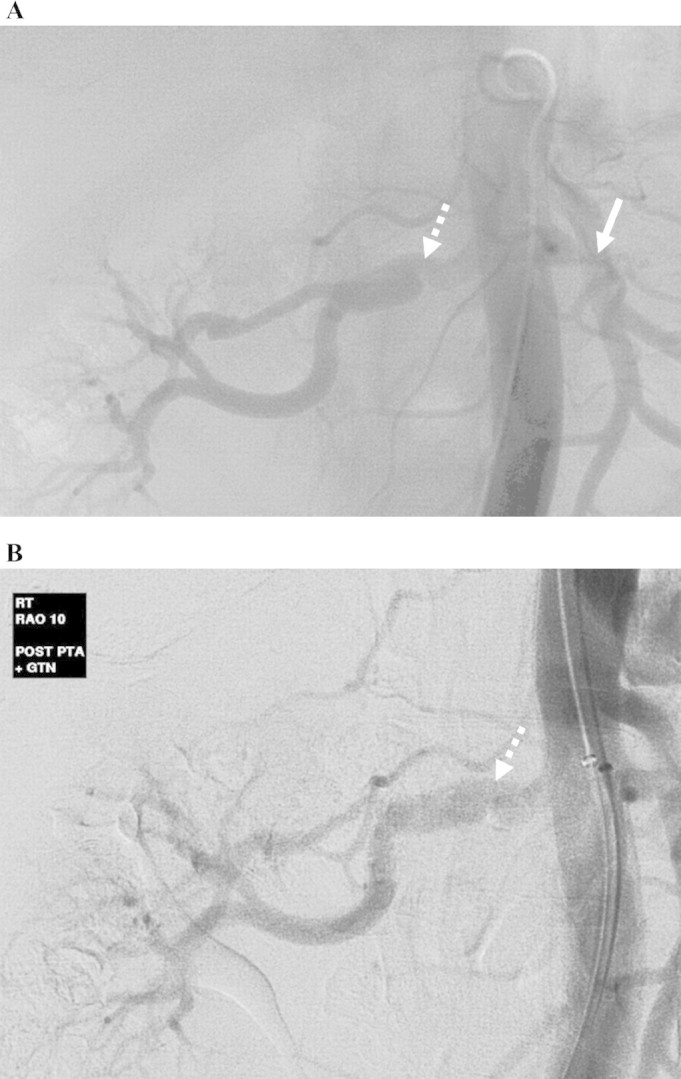
A 63-year-old man with diffuse atherosclerosis was admitted for elective angioplasty of the right renal artery. A previous CT renal angiogram revealed a critical stenosis of the right renal artery and occluded left renal artery with the shrunken left kidney. The right renal artery stenosis was pre-dilated with a 3-mm balloon and then stented with a 6 mm × 18 mm stent. There was a good immediate angiographic result (Figure 3A). However, the patient developed sudden acute right-sided loin pain 11 h after the procedure. His creatinine level rose from 190 μmol/L pre-operatively to 585 μmol/L the following day. His urine output also dropped to 55 mL in 24 h. An urgent CT renal angiogram was arranged that showed occlusion of the stent with thrombus extending just beyond the stent into the renal artery. An angiogram was performed the following morning that confirmed thrombosis of the right renal stent and several branches beyond the stent (Figure 3B). The right renal artery was then selectively catheterized and a bolus of 5 mg tPA was given followed by an infusion of 0.5 mg/h. Following a total 17 mg of tPA infusion, there was still some residual thrombus within the stent. Aspiration thrombectomy was then performed with several fragments of thrombus aspirated. This resulted in improved angiographic appearances but with a persistent filling defect at the distal end of the stent, consistent with an intimal flap, the likely cause of the original stent thrombosis (Figure 3C). This was therefore stented with a further 6 mm × 15 mm stent overlapping the original stent with a good angiographic result (Figure 3D). His renal function improved immediately afterwards eventually returning to baseline of 190 μmol/L and has been stable for the past 5 years.
Fig. 3.
Digital subtraction images of renal angiogram showing: (A) Good immediate angiographic result post-stent insertion of right renal artery. (B) Angiogram showing thrombosis of a stent and branched renal arteries (white arrow). (C) Resolution of thrombosis post-aspiration thrombectomy, with persisting filling defect at the distal end of the stent (white solid arrow), likely to represent an intimal flap. (D) Final appearance with a new stent overlapping the existing one.
Discussion
Acute renal infarction was not suspected initially in either of the first two cases, and the patients were managed initially with presumed diagnosis of renal calculi. In the first case, renal infarction was a result of thrombosis on an existing renal artery stenosis, most likely due to fibromuscular dysplasia. Intervention of the stenosis of the contralateral kidney together with regular surveillance will hopefully prevent it from thrombosis in the future. In the second case, the renal infarction was likely to be a result of thromboembolism, although no source of emboli could be found. In either case, the diagnosis was made too late to contemplate reperfusion therapy.
Reperfusion therapy should be considered in all suspected acute renal infarction, especially if the diagnosis is made early before irreversible ischaemic renal damage sets in. Successful renal reperfusion therapy has been reported with local intra-arterial thrombolysis and percutaneous rheolytic thrombectomy with or without angioplasty [3–5]. However, it is not known how long an ischaemic kidney can survive beyond which reperfusion therapy would be futile. Successful outcomes have been reported up to several days from the onset of symptoms, but arterial occlusion may have been incomplete in the late cases [3–5]. Our third case highlights that point that reperfusion therapy can be successful if treatment is initiated within a short time frame (up to 48 h in our case).
Renal infarction is a serious cause of acute nephron loss that is potentially reversible by reperfusion therapy. The diagnosis is often missed or delayed at presentation. This case report highlights the importance of considering renal infarction in the differential diagnosis in patients presenting with acute loin pain. The diagnosis can be established with various radiological techniques, including CT urogram, CT renal angiogram and DMSA radioisotope scan, depending on local availability. Definitive diagnosis and potential reperfusion therapy can be made with a formal renal angiogram. Expert opinion from nephrologists should be sought immediately if the diagnosis of acute renal infarction is suspected.
Conflict of interest statement. None declared.
References
- 1.Frost L, Engholm G, Johnsen S, et al. Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch Intern Med. 2001;161:272–276. doi: 10.1001/archinte.161.2.272. [DOI] [PubMed] [Google Scholar]
- 2.Korzets Z, Plotkin E, Bernheim J, et al. The clinical spectrum of acute renal infarction. Isr Med Assoc J. 2002;4:781–784. [PubMed] [Google Scholar]
- 3.Salam TA, Lumsden AB, Martin LG. Local infusion of fibrinolytic agents for acute renal artery thromboembolism: report of ten cases. Ann Vasc Surg. 1993;7:21–26. doi: 10.1007/BF02042655. [DOI] [PubMed] [Google Scholar]
- 4.Siablis D, Liatsikos EN, Goumenos D, et al. Percutaneous rheolytic thrombectomy for treatment of acute renal-artery thrombosis. J Endourol. 2005;19:68–71. doi: 10.1089/end.2005.19.68. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg JM, Steiner MA, Marshall JJ. Acute renal artery thrombosis treated by percutaneous rheolytic thrombectomy. Catheter Cardiovasc Interv. 2002;56:66–68. doi: 10.1002/ccd.10150. [DOI] [PubMed] [Google Scholar]