Sir,
Pathogenesis of steroid-sensitive nephrotic syndrome (SSNS) is thought to be related mainly to T-cell dysfunction [1]. However, the beneficial use of rituximab in cases of frequently relapsing SSNS provided evidence of B cell involvement [2–4]. Our aim was, thus, to investigate prospectively the levels of the circulating CD19+ B cells in children with a first episode of SSNS in sequential stages (presentation, remission on steroids and remission off steroids).
Twenty-three children (M/F = 13/10, age = 2.5–14 years, median = 4.32 years) with a first episode of SSNS were studied; 23/23 both at presentation (before steroids initiation) and in remission on steroids (40 mg/m2 on alternate day); 15/23 were tested as well in remission off steroids for at least 6 months. Twenty-five age-matched children who had come to the outpatient haematology clinic in order to be tested for b-thalassaemia trait were found to be negative and served as healthy controls (controls 1). Considering that the presentation of SSNS may be associated with a recent infection, mainly a respiratory tract infection, twenty age-matched children with an upper respiratory tract infection acted as a second control group (controls 2).
The percentages of CD3± T cells, as a pan T-cell marker, and the percentages of CD19+ and CD20± B cells were evaluated in all children. The above-mentioned parameters were determined in each sample by flow cytometry using the lysed whole blood method. The duochrome phycoerythrin-cyanin5 (PE-Cy5) conjugated CD3± monoclonal antibody (MoAb) and phycoerythrin (PE) conjugated CD19+ and CD20± MoAbs purchased from Beckman Coulter were used. The samples were analysed with a EPICS-XL flow cytometer. The results were expressed as percentages (%) of fluorescence-positive cells as well as actual numbers (cells/μL) of the circulating CD19+ and CD20+ B cells, based on the white blood cell count.
Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows (SPSS version 11.5). The paired t-test and independent-samples t-test were used to compare differences between study groups with and without paired data, respectively. Pearson's coefficient of correlation (r) was used to determine the correlations. A P ≤ 0.05 was considered to be statistically significant.
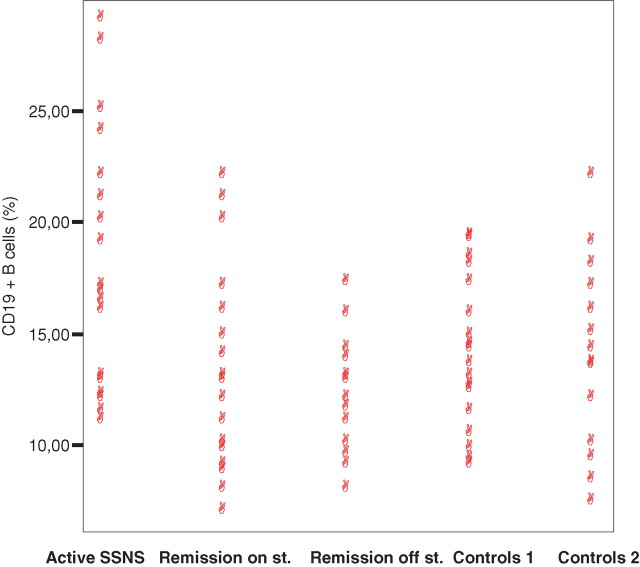
In 5 of 23 children with a first episode of SSNS, there was a recent history of an upper respiratory tract infection. Remission was achieved in all children within 6–15 days after steroid initiation. During remission, all patients presented normoalbuminaemia and were free of proteinuria and albuminuria. Percentages of CD3± T cells were found to be within normal limits in all patients (at presentation of SSNS, in remission still on steroids and in remission off steroids) compared with the two control groups (P ≥ 0.05). As depicted in Figure 1, the circulating CD19+ B cells were significantly higher at presentation of SSNS (mean percentage = 18.13 ± 5.43, mean actual number = 695.34 ± 258.29) compared with remission on steroids (mean percentage = 13.57 ± 4.22 and P < 0.0001, mean actual number = 468.05 ± 164.69 and P < 0.0001), remission off steroids (mean percentage = 13.25 ± 2.32 and P < 0.0001, mean actual number = 414.88 ± 140.76 and P < 0.0001), controls 1 (mean = 13.96 ± 3.29 and P = 0.008, mean actual number = 442.75 ± 99.78 and P = 0.009) and controls 2 (mean percentage = 14.18 ± 3.6 and P = 0.01, mean actual number = 508.05 ± 148.9 and P = 0.015). During remission stages, on and off steroids, CD19+ B cells were comparable with both control groups (P > 0.05). Moreover, circulating CD19+ B cells were inversely correlated with disease activity (r = −0.465, P < 0.0001). CD20+ B cells were similarly significantly higher at presentation of SSNS (mean percentage = 19.31 ± 4.24, mean actual number = 739.85 ± 267.34) compared with remission on steroids (mean percentage = 14.3 ± 3.92 and P < 0.0001, mean actual number = 493.17 ± 157.93 and P < 0.0001), remission off steroids (mean percentage = 13.25 ± 2.32 and P < 0.0001, mean actual number = 413.99 ± 142.64 and P < 0.0001), controls 1 (mean = 13.12 ± 4.01 and P = 0.001, mean actual number = 415.40.75 ± 167.34 and P = 0.002) and controls 2 (mean percentage = 13.5 ± 2.34 and P = 0.001, mean actual number = 483.63 ± 156.84 and P = 0.001). During remission stages, CD20+ B cells were comparable with both control groups (P > 0.05).
Fig. 1.
Percentage of CD19+ B cells in children with a first episode of SSNS in sequential stages (presentation, remission on steroids, remission off steroids) and in controls 1 and 2.
The pathogenesis of SSNS has mainly been focused on T cells dysfunction. The success of rituximab, a chimeric anti-CD20 antibody, in the treatment of cases of frequently relapsing SSNS initiated interest in pathogenic pathways involving B cells [2–4]. There is an interaction between B cells and T cells, and B cells are involved in the presentation of antigens to T cells. However, the effect of B-cell depletion on T-cell function is unknown. Kemper et al. [5] suggested that in children with SSNS, there is a combined T- and B-cell activation. In agreement with our results, recent studies showed that CD19+ B cells may be elevated in relapsing nephrotic children [6,7]. Children with both steroid-sensitive and steroid-resistant nephrotic syndrome were included in this study, and there were no paired data of the circulating CD19+ B cells in remission off steroids. It is worth noting that in our study not all children with SSNS presented elevated CD19+ B cells. We intend to follow up this cohort of 23 nephrotic children in order to identify whether the long-term outcome of the nephrotic syndrome is associated with the up-regulation of CD19+ B cells in these patients taking into account steroid dependency and frequency of relapses. Our findings demonstrated an up-regulation of circulating CD19+ and CD20± B cells in children with a first episode of SSNS. This indicates that B cells may play a role in SSNS and that rituximab may be considered in certain cases.
Conflict of interest statement. None declared.
References
- 1.Van De Berg J, Weening J. Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci. 2000;107:125–136. doi: 10.1042/CS20040095. [DOI] [PubMed] [Google Scholar]
- 2.François H, Daugas E, Bensman A, et al. Unexpected efficacy of rituximab in multirelapsing minimal change nephrotic syndrome in the adult: first case report and pathophysiological considerations. Am J Kidney Dis. 2007;49:158–161. doi: 10.1053/j.ajkd.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Hofstra JM, Deegens JK, Wetzels JF. Rituximab: effective treatment for severe steroid-dependent minimal change nephrotic syndrome? Nephrol Dial Transplant. 2007;22:2100–2102. doi: 10.1093/ndt/gfm128. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MS, Wong CF. Rituximab and nephrotic syndrome: a new therapeutic hope? Nephrol Dial Transplant. 2008;23:11–17. doi: 10.1093/ndt/gfm683. [DOI] [PubMed] [Google Scholar]
- 5.Kemper MJ, Meyer-Jark T, Lilova M, et al. Combined T- and B-cell activation in childhood steroid-sensitive nephrotic syndrome. Clin Nephrol. 2003;60:242–247. doi: 10.5414/cnp60242. [DOI] [PubMed] [Google Scholar]
- 6.Lama G, Luongo I, Tirino G, et al. T Lymphocyte populations and cytokines in childhood nephrotic syndrome. Am J Kidney Dis. 2002;39:958–965. doi: 10.1053/ajkd.2002.32769. [DOI] [PubMed] [Google Scholar]
- 7.Esposito Salsano M, Graziano L, Luongo I, et al. Atopy in childhood nephritic syndrome. Acta Paediatrica. 2007;96:561–566. doi: 10.1111/j.1651-2227.2007.00154.x. [DOI] [PubMed] [Google Scholar]