Abstract
Hyperacute rejection of a transplanted liver is rare even when the recipient has circulating donor-specific alloantibodies (DSA). There is also evidence that a transplanted liver may provide immunological protection for other organs transplanted from the same donor. We monitored the kinetics of circulating DSA in a highly sensitized recipient of a combined split liver and kidney transplant and demonstrated a reduction in antibody titres immediately after liver perfusion. The absorption of DSA was not compromised by the smaller liver mass transplanted. DSA titres remained low at 3 months post-transplant, and the recipient did not experience antibody-mediated rejection.
Keywords: HLA-specific antibody, hyperacute rejection, liver–kidney transplant
Background
In kidney transplantation, a positive complement-dependent lymphocytotoxic (CDC) crossmatch caused by antibodies directed against donor human leucocyte antigens (HLA) often results in hyperacute rejection [1]. However, hyperacute rejection of a transplanted liver is rare even when the recipient has circulating antibody directed against mismatched HLA or ABO blood group donor antigens [2]. There is evidence that the transplanted liver also provides immunological protection for other organs transplanted from the same donor [3]. Partial, auxiliary transplant of a liver to protect kidney grafts in highly sensitized patients has been described [4]. Various mechanisms have been proposed to explain the ability of the transplanted liver to absorb or neutralize donor-specific antibody (DSA) without succumbing to immediate graft dysfunction [5]. Depletion of HLA-specific antibodies post-transplant has been indirectly measured previously by the conversion of a positive crossmatch to negative in some cases [6]. This is the first report of the effects of a liver lobe transplant on the precise kinetics of HLA-specific antibody depletion in the intra- and post-operative period.
Case report
A 29-year-old female patient with congenital hepatic fibrosis and polycystic kidney disease received a combined liver lobe and kidney transplant donated after brain death. The recipient was consented for the procedure using our institution’s standard transplant information and consent form. The 31-year-old male donor was a 1.1.1 (HLA-A,-B,-DR) mismatch, and death was due to a subarachnoid haemorrhage. Both recipient and donor were ABO blood group A. The transplant procedure was relatively uncomplicated with the liver being implanted first. During the operative period, the patient received two units of packed red blood cells (a volume equating to ~ 700 mL) and 5 L of crystalloid fluids. The patient was given a single dose of methylprednisolone (500 mg) intra-operatively. Post-operative immunosuppression consisted of tacrolimus (trough level 10 μg/L) and prednisolone 20 mg per day. Mycophenolate mofetil (500 mg bd) was commenced at 3 months post-transplant.
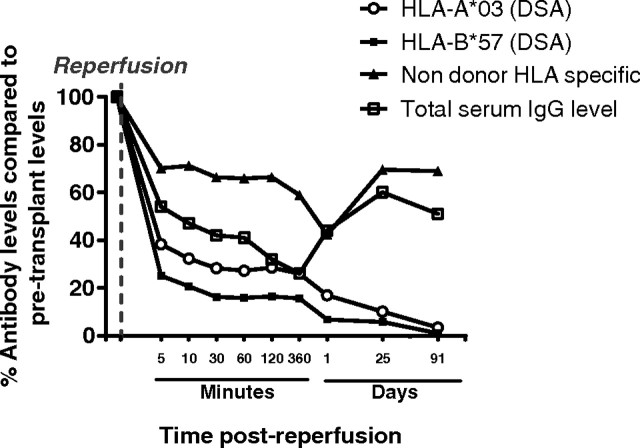
The recipient was nulliparous and had no previous transplants but was highly sensitized with multiple HLA class I- and II-specific antibodies, most likely caused by multiple blood transfusions from the age of 2 years. The pre-transplant CDC and flow cytometric crossmatch tests using T and B lymphocytes isolated from donor lymph nodes were IgG positive. The HLA specificities of the antibodies were characterized using a Luminex-based assay consisting of microbeads coated with purified recombinant HLA class I and II glycoproteins of a single HLA specificity (One Lambda Inc LABScreen® Single Antigen, analysed using a LABScan™ 100 flow analyser). In addition, total serum IgG levels were measured using nephelometry (BN™-II Analyser, Siemens Healthcare Diagnostics, Inc.). Analysis of the single antigen bead (SAB) results showed circulating IgG HLA class I-specific antibodies in recipient sera directed against mismatched donor HLA-A3 and HLA-B57. The levels of circulating HLA class I-specific antibodies were measured in sera samples taken at 5, 10 and 30 min; 1, 2 and 6 h; and 1, 25 and 91 days post-liver perfusion (Figure 1). Antibody monitoring showed a rapid decline in levels of circulating donor-specific antibodies within 5 min of liver reperfusion, such that levels of HLA-A3 were 38% and HLA-B57 25% of the immediate pre-transplant level. One day post-transplant, antibody levels had declined to 16% (HLA-A3) and 6% (HLA-B57) of the immediate pre-transplant levels.
Fig. 1.
HLA-specific and total serum IgG levels pre-liver transplant and at measured time intervals post-liver transplant reperfusion. The percentage decline in post-transplant levels, compared with pre-transplant baseline, for mismatched donor HLA-A*03 and HLA-B*57, non-donor HLA specificities and total serum IgG is shown.
Non-donor HLA-specific IgG levels (third party) showed a modest decline to 70% of the pre-transplant level at 5 min post-liver reperfusion, reducing to 43% at 1 h. Third party HLA-specific IgG levels recovered to 70% of pre-transplant levels at 1 and 3 months post-transplant. Total serum IgG levels were 54% at 5 min post-liver reperfusion and 26% at 1 h, recovering to 60% at 1 month.
There were no complications in the post-operative period, and both renal function and liver function were good at the time of discharge on Day 16 post-transplant. At 3 months post-transplant, the circulating levels of the donor-specific antibodies were < 5% of pre-transplant levels. Liver and kidney function tests were both within normal range at 8 months post-transplant, and there were no episodes of antibody-mediated rejection.
Discussion
The liver is unique in solid organ transplantation in its ability to resist hyperacute rejection and to protect other organs transplanted concomitantly from the same donor from rejection. Recipient selection is usually based on clinical urgency ABO blood group identity and size match without reference to HLA allosensitization status. Some transplant centres have also pursued a policy of transplanting a partial auxiliary liver graft with the sole purpose of protecting a transplanted kidney from hyperacute rejection in highly sensitized renal patients [4].
There are a number of theories for the apparent resistance to rejection and immune protective effect offered by the liver: the liver is a major source of soluble HLA class I molecules with reported immunomodulatory effects [7]; the mass of the liver is able to absorb antibody [5]; large numbers of passenger donor leucocytes are transferred with the graft and may establish a state of microchimerism and tolerance [8]; donor Kupffer cells lining the sinuses are replaced by recipient reticuloendothelial cells and may participate in the development of immune tolerance [9]; and finally, the liver is served with a dual blood supply which may protect the organ from ischaemic damage.
In the case we have presented, the recipient HLA sensitization status to the donor was established prior to liver transplantation allowing us to obtain serial intra- and post-operative blood samples for antibody monitoring using SAB.
This provided an opportunity to examine the kinetics of antibody depletion in the immediate post-transplant period. Our data indicate a rapid depletion of circulating donor-specific, HLA class I antibodies immediately following reperfusion of the transplanted liver lobe. The reduction in levels of non-donor HLA-specific antibody and total serum IgG indicates that the depletion of donor HLA-specific antibody was in part due to haemodilution as a result of the peri-operative administration of fluids. However, in comparison, the marked decline in DSA suggests a specific absorption beyond that caused by haemodilution alone. The rapid kinetics at which donor-specific antibody levels fell (within 5 min of reperfusion) suggests that antibody depletion in this context does not require de novo synthesis of a donor-specific neutralizing substance such as soluble HLA, but may reflect direct antibody binding to HLA glycoproteins on hepatic endothelial cells. The reduction in circulating DSA was not a temporary phenomenon but was still observed at 3 months, indicating an ongoing protective role. Clinically, this resulted in excellent long-term function of both allografts without antibody-mediated rejection.
In summary, our study confirms a rapid fall in DSA following hepatic reperfusion and suggests that concurrent transplant of a liver lobe may provide a realistic management option for highly sensitized patients awaiting solid organ transplantation.
Acknowledgments
This study is supported by the NIHR Cambridge Biomedical Research Centre.
Conflict of interest statement. None declared.
References
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Iwaki Y, Kano T, et al. Liver transplantation from crossmatch-positive donors. Transplant Proc. 1981;13:286–288. [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen A, Davies HF, Jamieson NV, et al. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 4.Olausson M, Mjörnstedt L, Nordén G, et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007;7:130–136. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 5.Fung JJ, Makowka L, Tzakis A, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20:88. [PMC free article] [PubMed] [Google Scholar]
- 6.Morrissey PE, Gordon F, Shaffer D, et al. Combined liver-kidney transplantation in patients with cirrhosis and renal failure: effect of a positive cross-match and benefits of combined transplantation. Liver Transplant Surg. 1998;4:363–369. doi: 10.1002/lt.500040512. [DOI] [PubMed] [Google Scholar]
- 7.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–747. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Wada T, Hoshino S, et al. Immunomodulatory role of Kupffer cell in liver allografts. Comp Hepatol. 2004;3:S32. doi: 10.1186/1476-5926-2-S1-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]