Abstract
It has been documented that preservation of residual renal function in dialysis patients improves quality of life as well as survival. Clinical trials on strategies to preserve residual renal function are clearly lacking. While waiting for more results from clinical trials, patients will benefit from clinicians being aware of available knowledge. The aim of this review was to offer an update on current evidence assisting doctors in clinical practice.
Keywords: evidence-based medicine, haemodialysis, peritoneal dialysis, residual renal function
Significance and measurement
Background
Initiating chronic dialysis treatment gives end-stage renal disease patients a new lease on life. However, the annual mortality rate in dialysis patients is ∼20 % [1], and quality of life is substantially reduced [2]. Observational studies have shown that preservation of residual renal function (RRF) in dialysis patients is a prognostic and independent factor in patient survival and quality of life [3, 4], also when RRF is almost completely lost [5].
A higher dialysis dose cannot compensate for declining RRF [6, 7]. The increased benefit of RRF compared to dialysis clearance is likely attributable to a better water and salt balance, the renal ability to clear and metabolize various substances including middle-sized molecules such as β-2-microglobulin and protein-bound substances [8] as well as the endocrine functions of the kidneys.
There is an increased focus on preserving RRF in dialysis patients [9], and factors affecting the decline of RRF are discussed in detail in this review and summarized in Figure 1.
Fig. 1.
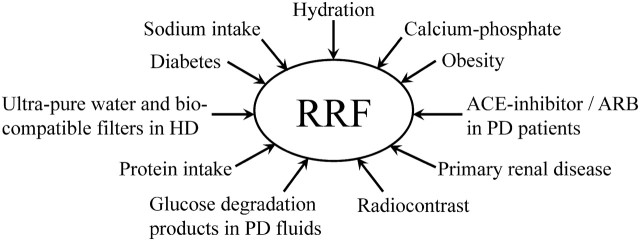
Factors assumed to affect the decline of residual renal function
Measurement methods
Glomerular filtration rate (GFR) is considered to be the best indicator of kidney function. However, determination of GFR in dialysis patients is not an easy task, and there is no gold standard. Consequently, measurements of RRF are inconsistently performed and reported in the literature.
Determination of GFR using exogene markers like 51Cr-EDTA or inulin is time consuming and labor intensive and has been scarcely investigated in dialysis patients.
Plasma concentrations of creatinine and urea are useless for GFR estimations in dialysis patients as these molecules are largely removed by dialysis treatment. Plasma concentrations of cystatin C [10] and beta-trace protein [11] have been proposed as new GFR markers in dialysis patients. Although promising, these middle-sized molecules have not been thoroughly investigated in different dialysis modalities. It is thus too early to implement these methods in clinical practice.
The most frequent method in clinical practice is 24-h urine collection and analysis of creatinine and/or urea in blood and urine. However, creatinine is secreted and urea is reabsorbed in the tubules, and these molecules can therefore not reflect GFR directly. Although the handling of creatinine and urea in the tubules is not related, the average value of creatinine and urea clearance is probably the best way to easily measure GFR.
Timing of RRF measurements
Peritoneal dialysis (PD) patients in a stable condition supposedly have small fluctuations in plasma level of creatinine and urea, at least when a continuous regimen is used. A 24-h urine collection can therefore be performed at a random day. In contrast, haemodialysis (HD) patients are not in a steady state as waste is removed intensively during dialysis sessions with a slower accumulation between sessions. Some clinicians advocate urine to be collected in the entire interdialytic interval. However, studies indicate that urine collections from the last 24 h before a dialysis session are rather reproducible [12, 13]. Measurement of RRF will guide dietary advice, dialysis dosing and medical treatment.
Renal disease and comorbidity
Primary renal disease
The RRF decline is significantly higher in diabetic patients as well as in patients with a glomerular disease compared to a tubulointerstitial kidney disease [14]. Patients with adult polycystic kidney disease generally preserve RRF for a longer time than other patient groups [15].
Patients entering dialysis treatment after loss of renal graft function tend to lose RRF faster than other patients initiating dialysis. Some low-grade immunosuppression of transplanted patients and possibly also patients with vasculitis-mediated renal disease may be justified to keep RRF and consequently improve life expectancy, although immunosuppression increases the risk of cancer and infection [16, 17].
Comorbidity
Cardiovascular disease (CVD) is the most common comorbid condition in dialysis patients. However, CVD and risk factors are different in dialysis patients compared to other ischaemic patients, and treatment of risk factors is controversial. Dialysis patients with stenosis of the renal arteries may benefit from revascularization [18]. Chronic heart failure is another prerenal factor predisposing to RRF decline. Optimal treatment e.g. with telmisartan [19] and perhaps revascularization of the coronary arteries might increase cardiac output leading to an increase in renal blood flow and thereby GFR.
Obesity was recently found to be a strong risk factor in the decline of RRF after initiation of dialysis therapy [20]. On the other hand, higher body mass index is associated with reduced mortality, at least in HD patients [21].
Elevated uric acid was linked to a faster decline in RRF among PD patients [22], but the long-term effects of intervention to reduce uric acid have not been studied.
Declining RRF is inevitable in most dialysis patients due to progression of the primary renal disease, but an increased focus on e.g. obstructive renal disease, urinary tract infection, diabetes mellitus regulation and possibly smoking cessation could contribute to the preservation of RRF.
Dialysis
Initiation of dialysis
It has been speculated that starting dialysis early might improve the condition and life expectancy of renal patients. However, previous studies as well as the IDEAL study reported no survival benefit of starting dialysis therapy at a higher GFR level [23]. This could probably be attributed to either a deleterious effect on RRF of dialysis per se or to the necessary interventions and complications occurring at the start of dialysis. In a randomized study of patients aged ≥70 years, a very low-protein diet could postpone initiation of dialysis and fewer hospitalizations were seen. There was also a tendency to better survival [24]. RRF was not measured in these studies, and, consequently, we do not know if early versus late initiation of dialysis affects the decline of RRF. We suggest initiating dialysis based on symptoms, not GFR.
Dialysis techniques
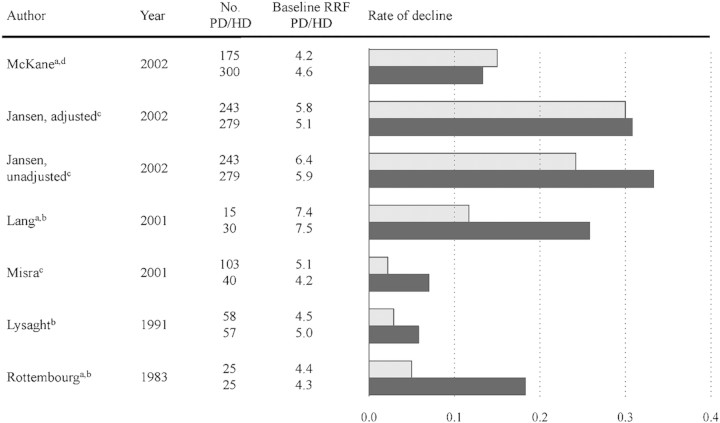
Although no randomized studies have been successful in clarifying the differences in loss of RRF in HD and PD, it is widely held that patients in HD lose RRF more rapidly than PD patients [25]. However, PD patients are more dependent on RRF than HD patients due to the limitation of dialysis dose in PD. Patients will therefore be initiated on HD or transferred from PD to HD if the decline in RRF is rapid, thereby causing selection bias. Consequently, observational studies comparing the effect of HD and PD on RRF are difficult to interpret [26]. During the first year of dialysis, the decline in RRF is reported to be as diverse as 0.18–0.33 mL/min/month in HD patients and 0.05–0.30 mL/min/month in PD patients [27], Figure 2.
Fig. 2.
Decline of RRF in PD (gray bar) and HD (black bar) patients during the first 12 months of dialysis treatment. Baseline RRF and rate of decline are measured in milliliter per minute and milliliter per minute per month, respectively. aDecline rates are not given in the article but calculated from residual renal function values at baseline and 12 months. bRRF estimated as creatinine clearance. cRRF estimated as (creatinine and urea clearance)/2. dRRF estimated as urea clearance.
In contrast to GFR, urine output in patients with end-stage renal disease ranges from oliguria to normal levels. Volume expansion in combination with urea osmotic diuresis and impaired ability to regulate sodium and water excretion contribute to maintaining urinary output in pre-dialysis patients [28]. However, both volume expansion and the high urea load per nephron are reversed by dialysis. This could explain why some, especially HD patients, have a marked reduction, or even cessation, of urine output shortly after initiation of dialysis treatment. Moreover, this may explain the differences between PD and HD during the first year of treatment, as PD patients are probably more overhydrated than HD patients.
HD patients seem to benefit from dialysis with ultrapure water, bicarbonate buffer and biocompatible membranes [29, 30]. Possible effects of convective therapy modalities with haemodiafiltration and haemofiltration have not been investigated in detail, and little is known regarding RRF in home HD patients. For PD patients, a difference in RRF decline between the continuous form (CAPD) and intermittent PD has been claimed, but not confirmed [31]. PD solutions containing fewer glucose degradation products might [32] or might not [33] protect RRF.
In general, dialysis techniques have improved making comparison of older and newer studies difficult.
Inflammation
A strong association between C-reactive protein level and RRF loss has been reported in the first year of PD [34]. In a study on HD patients without clinical infection, circulating bacteria-derived DNA was linked to increased levels of C-reactive protein and interleukin-6 [35]; effects on RRF were not reported.
A pilot study in PD patients indicated that N-acetylcysteine may be able to preserve RRF [36]. Physical training is shown to decrease levels of C-reactive protein [37] but has no measurable effects on circulating cytokines [38]. Effect on RRF was not assessed. Further studies elucidating inflammation pathways in dialysis patients as well as studies reporting whether anti-inflammatory strategies can affect hard end points are warranted.
Diet
Protein intake
Adult dialysis patients are recommended to eat a diet containing ∼1.2 g/kg body weight of proteins per day as opposed to non-dialysis patients with GFR <30 mL/min/1.73m2 where 0.60–0.75 g/kg is recommended [39]. Variable amounts of protein are lost during dialysis. Continuous protein loading may cause hyperfiltration in the kidneys [40] in addition to an increased load of phosphate, urea and acid. This might support diuresis in the short term but could result in a faster RRF decline over time. In a study of incident PD patients, 60 individuals were randomized to protein intakes of 0.6–0.8 g/kg, 0.6–0.8 g/kg + keto acids or the usual high-protein diet of 1.0–1.2 g/kg. After 1 year, the group receiving 0.6–0.8 g/kg + keto acids did not experience any decline in RRF, which was the case in the other two groups [41]. In another PD study, patients continued a protein- and sodium-restricted diet while the effects of an angiotensin II receptor blocker (ARB) on RRF were tested in an open labelled study. Low RRF declining rates were demonstrated in both patient groups [42]. In HD patients, no randomized studies of protein intake have been published, but a recent observational study revealed that a protein intake <1.2 g/kg was associated with increased mortality [43]; this could possibly be partly explained by poor nutrition.
In general, not many dialysis patients consume the recommended amount of protein. Protein restriction seems to protect RRF in PD patients. However, a low protein intake is often linked to a low energy intake possibly leading to malnutrition. Assistance from a dietician experienced in dialysis patients may be helpful. Further studies are needed to elucidate the optimal protein intake in dialysis patients.
Fluid and salt intake
Intake of salt and fluid plays an important role in end-stage renal disease. As RRF declines, salt and fluid restriction becomes an increasing burden, and in anuria, an intake of 9 g of sodium chloride needs 1 L of water to keep osmolarity unchanged.
Overhydration is linked to increased blood pressure and cardiac workload [44]. Moreover, overhydration can possibly assist diuresis, whereas effects on GFR are questionable. On the other hand, intravascular volume depletion can adversely affect RRF [27], possibly due to prerenal and ischaemia-induced acute kidney injury. More frequent and/or longer dialysis treatments are desirable [45] and lower dry weight could thus be reached slowly without intravascular volume depletion. Furthermore, dialysis patients could benefit from instructions to drink more and control their weight during fevers or very hot weather to reduce the number of dehydration episodes.
However, the fluid and salt issue is confused by a study reporting that a low dietary sodium intake independently predicts overall and cardiovascular mortality in PD patients [46]. In this study, low sodium intake was associated with declining RRF, although the differences between tertiles did not reach statistical significance.
Keeping dialysis patients normohydrated is desirable, and better methods are needed to determine optimal dry weight.
Medications
Blood pressure control and drug use
Current American guidelines advocates ‘target blood pressure for CVD risk reduction in chronic kidney disease (CKD) should be <130/80 mmHg’ [47]. However, it is not specified whether this target also concerns dialysis patients. No randomized studies on the effects of different blood pressure levels in dialysis patients are available. Thus, the optimum blood pressure is unknown. Moreover, it is disputed if blood pressure is to be measured ambulatory, pre-dialysis or post-dialysis.
Prospective trials in PD patients have suggested that ramipril (an ACE inhibitor) and valsartan (an ARB) can protect RRF [42, 48]. These results cannot be extended to HD patients; primarily because blockage of the renin–angiotensin system (RAS) may increase the risk of intradialytic hypotensive episodes possibly causing ischaemia-induced kidney damage. In addition, ACE inhibitors and ARBs may elevate potassium levels, although a recent study showed that neither monotherapy (ACE inhibitor or ARB) nor combination therapy (ACE inhibitor plus ARB) was associated with additional risk of hyperkalaemia in patients on maintenance HD, except in anuric patients [49]. One study has reported a beneficial effect of calcium channel blockers on RRF in PD patients (but not HD patients) [50]; another study on PD patients was reported to have a detrimental effect on RRF [51]. The effect of beta-blockage on RRF is unknown.
The OCTOPUS study [52] is a prospective randomized controlled study among hypertensive HD patients in Japan. Different antihypertensive treatment regimens are compared, including RAS inhibition and antihypertensive drugs without RAS inhibitors. However, this study does not address the question of RRF.
In Tassin, HD patients are dialysed 8–9 h three times per week and only 3–5% of the patients need antihypertensive medications. Effects on RRF are not reported [53].
Further studies of optimum blood pressure level as well as nonpharmacologic and pharmacologic treatment of elevated blood pressure in dialysis patients are highly needed.
Loop diuretics
Short-term studies in patients on CAPD and HD show that large doses of loop diuretics (500–2000 mg/day) can increase urine volume plus sodium and potassium excretion [54, 55]. No effect in patients with urine output < 100 mL/day was documented, and the magnitude of the natriuresis was greater in patients with higher GFR. However, short-term studies tell nothing about preservation of RRF.
In 61 CAPD patients, a long-term study revealed that a furosemide dose of 250 mg/day had no effect on the rate of decline of RRF but led to a clinically significant preservation of urine volume after 1 year. There was no observed side effects and the treatment resulted in a possible improvement in hydration [56]. Preservation of urine volume was also seen in a Dialysis Outcomes and Practice Patterns Study (DOPPS) analysis [57]. Treatment with 250–1000 mg of furosemide in 13 HD patients documented a marked initial rise in fluid and electrolyte excretion with a graduated decrease in response during 1 year, possibly due to progression of primary renal disease [55]. On the other hand, high-dose loop diuretics can have side effects such as sunlight-induced bullae on the skin and ototoxicity [58].
Phosphate binders
In a rat model, low-phosphate diets showed protection of RRF [59] regardless of dietary protein content [60]. This is supported by a study in humans demonstrating that a high phosphate level was a risk factor for RRF decline and mortality in pre-dialysis patients [61]. However, in an observational study on HD and PD patients, a modest disordered mineral metabolism could not be demonstrated to affect RRF decline [62].
No randomized trials in humans have elucidated if low phosphate blood levels can protect RRF or which phosphate binder to use [63].
Radiocontrast
In PD patients, a temporary decline in RRF was demonstrated after use of radiocontrast [64]. However, decline in RRF was not different in treated groups compared to controls 2–4 weeks after contrast administration in another group of PD patients [65] or after 6 months in HD patients [66]. None of the patients participating in these three studies received N-acetylcysteine. PD patients were hydrated with 0.5–1 L of water, whereas HD patients followed their normal regime and were dialysed 3–5 h after the investigation. Although radiocontrast agents are easily removed with HD, patients with advanced renal insufficiency may [67] or may not [68] benefit from HD treatment immediately after exposure. Adequate hydration may prevent a possible deleterious effect of radiocontrast as aggressive furosemide treatment in patients with CKD (not on dialysis) resulted in added toxicity of radiocontrast agents [69].
Despite these facts, investigations involving iodinated contrast agents are often postponed or not performed with the intention to preserve RRF.
Other potential nephrotoxic drugs
As suggested above, low-grade immunosuppression may be justified to retain some RRF when renal transplant patients start dialysis. Without evidence, stopping the calcineurin inhibitor at the start of dialysis seems to be the best choice to alleviate the chronic fibrosis and acute hemodynamic effects of the calcineurin inhibitors and thus maintain the highest possible levels of RRF. Mycophenolate mofetil and/or prednisolone can be continued.
Other drugs like aminoglycosides may adversely affect RRF [70, 71]. However, a study in 102 PD patients was unable to demonstrate that a course of aminoglycoside reaching 14 days was deleterious to RRF compared to cephalosporin treatment [72].
Non-steroidal anti-inflammatory drugs (NSAID) are sometimes avoided due to unwanted acute effects on RRF and cardiovascular side effects, although a DOPPS analysis did not reveal any effect of NSAID on RRF [73].
Conclusions
The main limitation in the field of RRF is that all data linking preserved RRF to survival have been collected from observational studies. However, preservation of RRF is surely to the benefit of the patient. Awareness of the possibilities to preserve RRF listed above is justified, although only few robust randomized controlled trials support the recommendations suggested. Improved and standardized methods for estimation of RRF in PD as well as HD patients are desirable.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 2.Sondergaard H, Juul S. Self-rated health and functioning in patients with chronic renal disease. Dan Med Bull. 2010;57:A4220. [PubMed] [Google Scholar]
- 3.Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao CT, Chen YM, Shiao CC, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009;24:2909–2914. doi: 10.1093/ndt/gfp056. [DOI] [PubMed] [Google Scholar]
- 5.van der Wal WM, Noordzij M, Dekker FW, et al. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfq856. doi 10.1093/ndt/gfq856 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Vilar E, Wellsted D, Chandna SM, et al. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24:2502–2510. doi: 10.1093/ndt/gfp071. [DOI] [PubMed] [Google Scholar]
- 7.Termorshuizen F, Korevaar JC, Dekker FW, et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis. 2003;41:1293–1302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 8.Bargman JM, Thorpe KE, Churchill DN, et al. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 9.Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068–1081. doi: 10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Hoek FJ, Korevaar JC, Dekker FW, et al. Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant. 2007;22:1633–1638. doi: 10.1093/ndt/gfm027. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt T, Poge U, Stoffel-Wagner B, et al. Serum levels of beta-trace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant. 2008;23:309–314. doi: 10.1093/ndt/gfm510. [DOI] [PubMed] [Google Scholar]
- 12.van Olden RW, van Acker BA, Koomen GC, et al. Time course of inulin and creatinine clearance in the interval between two haemodialysis treatments. Nephrol Dial Transplant. 1995;10:2274–2280. doi: 10.1093/ndt/10.12.2274. [DOI] [PubMed] [Google Scholar]
- 13.Milutinovic J, Cutler RE, Hoover P, et al. Measurement of residual glomerular filtration rate in the patient receiving repetitive hemodialysis. Kidney Int. 1975;8:185–190. doi: 10.1038/ki.1975.98. [DOI] [PubMed] [Google Scholar]
- 14.Iest CG, Vanholder RC, Ringoir SM. Loss of residual renal function in patients on regular haemodialysis. Int J Artif Organs. 1989;12:159–164. [PubMed] [Google Scholar]
- 15.Van Stone JC. The effect of dialyzer membrane and etiology of kidney disease on the preservation of residual renal function in chronic hemodialysis patients. ASAIO J. 1995;41:M713–M716. doi: 10.1097/00002480-199507000-00105. [DOI] [PubMed] [Google Scholar]
- 16.Davies SJ. Peritoneal dialysis in the patient with a failing renal allograft. Perit Dial Int. 2001;21(Suppl 3):S280–S284. [PubMed] [Google Scholar]
- 17.Jassal SV, Lok CE, Walele A, et al. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: results of a decision analysis. Am J Kidney Dis. 2002;40:178–183. doi: 10.1053/ajkd.2002.33927. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer JP, Greco BA, Lewis JB. Evaluation of renal artery stenosis in dialysis patients. Semin Dial. 2009;22:519–523. doi: 10.1111/j.1525-139X.2009.00618.x. [DOI] [PubMed] [Google Scholar]
- 19.Cice G, Di Benedetto A, D'Isa S, et al. Effects of telmisartan added to angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010;56:1701–1708. doi: 10.1016/j.jacc.2010.03.105. [DOI] [PubMed] [Google Scholar]
- 20.Drechsler C, de Mutsert R, Grootendorst DC, et al. Association of body mass index with decline in residual kidney function after initiation of dialysis. Am J Kidney Dis. 2009;53:1014–1023. doi: 10.1053/j.ajkd.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 22.Park JT, Kim DK, Chang TI, et al. Uric acid is associated with the rate of residual renal function decline in peritoneal dialysis patients. Nephrol Dial Transplant. 2009;24:3520–3525. doi: 10.1093/ndt/gfp272. [DOI] [PubMed] [Google Scholar]
- 23.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 24.Brunori G, Viola BF, Parrinello G, et al. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: a prospective randomized multicenter controlled study. Am J Kidney Dis. 2007;49:569–580. doi: 10.1053/j.ajkd.2007.02.278. [DOI] [PubMed] [Google Scholar]
- 25.Tam P. Peritoneal dialysis and preservation of residual renal function. Perit Dial Int. 2009;29(Suppl 2):S108–S110. [PubMed] [Google Scholar]
- 26.Misra M, Vonesh E, Churchill DN, et al. Preservation of glomerular filtration rate on dialysis when adjusted for patient dropout. Kidney Int. 2000;57:691–696. doi: 10.1046/j.1523-1755.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 27.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 28.Yeh BP, Tomko DJ, Stacy WK, et al. Factors influencing sodium and water excretion in uremic man. Kidney Int. 1975;7:103–110. doi: 10.1038/ki.1975.15. [DOI] [PubMed] [Google Scholar]
- 29.McKane W, Chandna SM, Tattersall JE, et al. Identical decline of residual renal function in high-flux biocompatible hemodialysis and CAPD. Kidney Int. 2002;61:256–265. doi: 10.1046/j.1523-1755.2002.00098.x. [DOI] [PubMed] [Google Scholar]
- 30.Schiffl H, Lang SM, Fischer R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant. 2002;17:1814–1818. doi: 10.1093/ndt/17.10.1814. [DOI] [PubMed] [Google Scholar]
- 31.Rabindranath KS, Adams J, Ali TZ, et al. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2007;22:2991–2998. doi: 10.1093/ndt/gfm515. [DOI] [PubMed] [Google Scholar]
- 32.Haag-Weber M, Kramer R, Haake R, et al. Low-GDP fluid (Gambrosol trio(R)) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant. 2010;25:2288–2296. doi: 10.1093/ndt/gfq087. [DOI] [PubMed] [Google Scholar]
- 33.Fan SL, Pile T, Punzalan S, et al. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 2008;73:200–206. doi: 10.1038/sj.ki.5002574. [DOI] [PubMed] [Google Scholar]
- 34.Chung SH, Heimburger O, Stenvinkel P, et al. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol Dial Transplant. 2001;16:2240–2245. doi: 10.1093/ndt/16.11.2240. [DOI] [PubMed] [Google Scholar]
- 35.Bossola M, Sanguinetti M, Scribano D, et al. Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:379–385. doi: 10.2215/CJN.03490708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldman L, Shani M, Efrati S, et al. N-acetylcysteine improves residual renal function in peritoneal dialysis patients: a pilot study. Perit Dial Int. 2010 doi: 10.3747/pdi.2009.00263. [DOI] [PubMed] [Google Scholar]
- 37.Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 38.Cheema BS, Abas H, Smith BC, et al. Effect of resistance training during hemodialysis on circulating cytokines: a randomized controlled trial. Eur J Appl Physiol. 2010 doi: 10.1007/s00421-010-1763-5. [DOI] [PubMed] [Google Scholar]
- 39.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(1 Suppl 2):S66–S70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 40.Woods LL. Mechanisms of renal hemodynamic regulation in response to protein feeding. Kidney Int. 1993;44:659–675. doi: 10.1038/ki.1993.299. [DOI] [PubMed] [Google Scholar]
- 41.Jiang N, Qian J, Sun W, et al. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: a prospective, randomized trial. Nephrol Dial Transplant. 2009;24:2551–2558. doi: 10.1093/ndt/gfp085. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki H, Kanno Y, Sugahara S, et al. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis. 2004;43:1056–1064. doi: 10.1053/j.ajkd.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Antunes AA, Delatim Vannini F, de Arruda Silveira LV, et al. Influence of protein intake and muscle mass on survival in chronic dialysis patients. Ren Fail. 2010;32:1055–1059. doi: 10.3109/0886022X.2010.510233. [DOI] [PubMed] [Google Scholar]
- 44.Tian JP, Du FH, Cheng LT, et al. Residual renal function and arterial stiffness mediated the blood pressure change during interdialytic weight gain in hemodialysis patients. Hemodial Int. 2009;13:479–486. doi: 10.1111/j.1542-4758.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- 45.Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong J, Li Y, Yang Z, et al. Low dietary sodium intake increases the death risk in peritoneal dialysis. Clin J Am Soc Nephrol. 2010;5:240–247. doi: 10.2215/CJN.05410709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1–S290. [PubMed] [Google Scholar]
- 48.Li PK, Chow KM, Wong TY, et al. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med. 2003;139:105–112. doi: 10.7326/0003-4819-139-2-200307150-00010. [DOI] [PubMed] [Google Scholar]
- 49.Han SW, Won YW, Yi JH, et al. No impact of hyperkalaemia with renin-angiotensin system blockades in maintenance haemodialysis patients. Nephrol Dial Transplant. 2007;22:1150–1155. doi: 10.1093/ndt/gfl752. [DOI] [PubMed] [Google Scholar]
- 50.Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 51.Borrego F, Selgas R, de Alvaro F, et al. Seguimiento de la función renal residual en pacientes en diálisis peritoneal continua ambulatoria (DPCA): la influencia de los aclaramientos peritoneales y de los fármacos. Nefrologia. 1993;13:37–46. [Google Scholar]
- 52.Iseki K, Tokuyama K, Shiohira Y, et al. Olmesartan clinical trial in Okinawan patients under OKIDS (OCTOPUS) study: design and methods. Clin Exp Nephrol. 2009;13:145–151. doi: 10.1007/s10157-008-0116-8. [DOI] [PubMed] [Google Scholar]
- 53.Charra B, Terrat JC, Vanel T, et al. Long thrice weekly hemodialysis: the Tassin experience. Int J Artif Organs. 2004;27:265–283. doi: 10.1177/039139880402700403. [DOI] [PubMed] [Google Scholar]
- 54.Krediet RT, Douma CE, van Olden RW, et al. Augmenting solute clearance in peritoneal dialysis. Kidney Int. 1998;54:2218–2225. doi: 10.1046/j.1523-1755.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 55.van Olden RW, van Meyel JJ, Gerlag PG. Acute and long-term effects of therapy with high-dose furosemide in chronic hemodialysis patients. Am J Nephrol. 1992;12:351–356. doi: 10.1159/000168471. [DOI] [PubMed] [Google Scholar]
- 56.Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59:1128–1133. doi: 10.1046/j.1523-1755.2001.0590031128.x. [DOI] [PubMed] [Google Scholar]
- 57.Bragg-Gresham JL, Fissell RB, Mason NA, et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS) Am J Kidney Dis. 2007;49:426–431. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Sica DA, Gehr TW. Diuretic use in stage 5 chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2003;12:483–490. doi: 10.1097/00041552-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Koizumi T, Murakami K, Nakayama H, et al. Role of dietary phosphorus in the progression of renal failure. Biochem Biophys Res Commun. 2002;295:917–921. doi: 10.1016/s0006-291x(02)00793-3. [DOI] [PubMed] [Google Scholar]
- 60.Kusano K, Segawa H, Ohnishi R, et al. Role of low protein and low phosphorus diet in the progression of chronic kidney disease in uremic rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:237–243. doi: 10.3177/jnsv.54.237. [DOI] [PubMed] [Google Scholar]
- 61.Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 62.Noordzij M, Voormolen NM, Boeschoten EW, et al. Disordered mineral metabolism is not a risk factor for loss of residual renal function in dialysis patients. Nephrol Dial Transplant. 2009;24:1580–1587. doi: 10.1093/ndt/gfn768. [DOI] [PubMed] [Google Scholar]
- 63.Navaneethan SD, Palmer SC, Craig JC, et al. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. 2009;54:619–637. doi: 10.1053/j.ajkd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant. 2006;21:1334–1339. doi: 10.1093/ndt/gfi023. [DOI] [PubMed] [Google Scholar]
- 65.Moranne O, Willoteaux S, Pagniez D, et al. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant. 2006;21:1040–1045. doi: 10.1093/ndt/gfi327. [DOI] [PubMed] [Google Scholar]
- 66.Janousek R, Krajina A, Peregrin JH, et al. Effect of intravascular iodinated contrast media on natural course of end-stage renal disease progression in hemodialysis patients: a prospective study. Cardiovasc Intervent Radiol. 2010;33:61–66. doi: 10.1007/s00270-009-9715-3. [DOI] [PubMed] [Google Scholar]
- 67.Lee PT, Chou KJ, Liu CP, et al. Renal protection for coronary angiography in advanced renal failure patients by prophylactic hemodialysis. A randomized controlled trial. J Am Coll Cardiol. 2007;50:1015–1020. doi: 10.1016/j.jacc.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 68.Vogt B, Ferrari P, Schonholzer C, et al. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am J Med. 2001;111:692–698. doi: 10.1016/s0002-9343(01)00983-4. [DOI] [PubMed] [Google Scholar]
- 69.Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 70.Shemin D, Maaz D, St Pierre D, et al. Effect of aminoglycoside use on residual renal function in peritoneal dialysis patients. Am J Kidney Dis. 1999;34:14–20. doi: 10.1016/s0272-6386(99)70102-2. [DOI] [PubMed] [Google Scholar]
- 71.Singhal MK, Bhaskaran S, Vidgen E, et al. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int. 2000;20:429–438. [PubMed] [Google Scholar]
- 72.Lui SL, Cheng SW, Ng F, et al. Cefazolin plus netilmicin versus cefazolin plus ceftazidime for treating CAPD peritonitis: effect on residual renal function. Kidney Int. 2005;68:2375–2380. doi: 10.1111/j.1523-1755.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 73.Bailie GR, Mason NA, Bragg-Gresham JL, et al. Analgesic prescription patterns among hemodialysis patients in the DOPPS: potential for underprescription. Kidney Int. 2004;65:2419–2425. doi: 10.1111/j.1523-1755.2004.00658.x. [DOI] [PubMed] [Google Scholar]