Abstract
A patient presented with cardiac failure and investigations revealed a right femoral arteriovenous fistula (AVF) 5 weeks after the insertion of a femoral dialysis catheter for haemodialysis. No ultrasound had been used for catheter insertion. The iatrogenic AVF was repaired, and her episodes of cardiac failure ceased. It is recommended that femoral dialysis catheters are inserted using ultrasound guidance to avoid inadvertent arterial puncture.
Keywords: arteriovenous fistula, cardiac failure, dialysis access, iatrogenic, surgery
Introduction
Percutaneous transfemoral arterial catheterization is commonly preferred as the mode of access for a multitude of diagnostic and interventional procedures by cardiologists and interventional radiologists. Percutaneous femoral venous catheterization is often used by intensivists and nephrologists for temporary haemodialysis access. Puncture site complications can occur, most frequently haemorrhage, pseudoaneurysm formation, dissection with arterial thrombosis and distal thromboembolism [1].
Iatrogenic arteriovenous fistula (AVF) is rare. Femoral AVFs can result from penetrating injuries as well as more frequently from percutaneous vascular interventions. The incidence of post-catheterization AVFs ranges from 0.006 to 0.86% whilst that of all vascular complications varies from 1 to 9% [1–4]. AVFs in the groin frequently result from low groin punctures. The lack of bony support during compression post-catheter removal results in difficulty in achieving haemostasis. This is further compounded by the anatomical positions of the common femoral vein lying almost behind the superficial femoral artery.
We report a case of an iatrogenic AVF resulting from placement of a temporary dialysis femoral line in the groin leading to worsening of cardiac failure.
Case report
A 53-year-old lady in established renal failure secondary to Alport's syndrome had been on haemodialysis for 6 years using a left brachio-cephalic AVF. Medications included dual anti-platelet therapy and a once daily dose of low-molecular-weight heparin. A right femoral dialysis catheter was inserted for haemodialysis at a district hospital where she presented with florid pulmonary oedema, rather than using the AVF for reasons that remain unclear. The procedure was apparently traumatic and painful with considerable bleeding upon removal of the catheter 2 weeks later.
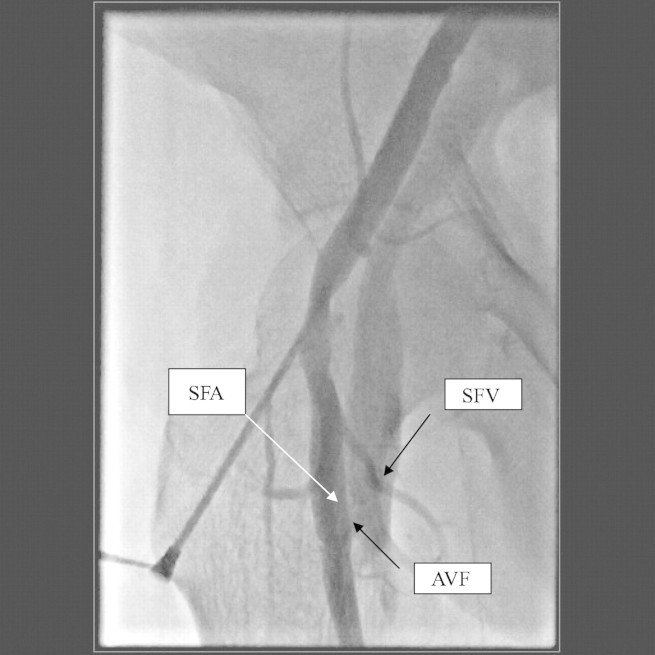
Subsequently she developed cardiac failure and was referred to cardiology for management of what was thought to be cardiac insufficiency. A transthoracic echocardiogram revealed moderate mitral stenosis and regurgitation with a hyperdynamic left ventricular function. Acute coronary syndrome was suspected, and cardiac catheterization performed through the same groin. The coronary angiographic study revealed moderate non-occlusive right coronary artery atherosclerosis. However, a femoro–femoral AVF was discovered (Figure 1). It was postulated that the high output state was made worse by the iatrogenic femoral AVF.
Fig. 1.
Angiography revealing the arteriovenous communication (between the superficial femoral artery and superficial femoral vein).
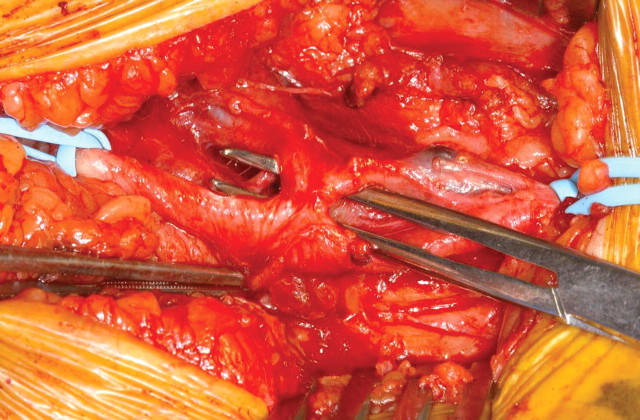
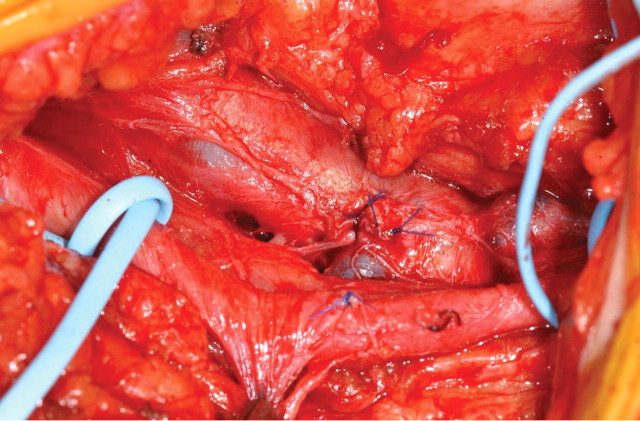
Clinical assessment following referral for surgery revealed a palpable thrill and an audible bruit on auscultation in the affected groin. Surgical exploration revealed a communication between the superficial femoral artery (SFA) and the superficial femoral vein (SFV) (Figure 2). The SFA and SFV were gently separated after full arterial and venous control, and a primary closure of both defects in the artery and vein was then carried out with running non-absorbable sutures (Figure 3). The post-operative period was uneventful, and the patient discharged 2 days later.
Fig. 2.
Intra-operative exposure of the fistulous connection (artery is slung proximal and distal to the fistula).
Fig. 3.
Appearance after repair.
Discussion
AVF represents an abnormal communication occurring secondary to penetrating injuries, rarely in blunt trauma [5] or as a result of percutaneous interventions, surgical procedures and venous catheterization of the extremities.
Diagnosis can be achieved by history and clinical examination, and this can be complemented by imaging modalities such as colour-coded Doppler ultrasound (duplex ultrasonography), CT or MR imaging as well as conventional angiography for accurate localization of the AVF [1,6,7] although duplex scan is the simplest and least invasive method.
Surgical repair remains the gold standard in which the fistulous tract is identified, resected and primary repair performed. This offers a 96% chance of definitive closure [8]. Potential complications of surgical intervention are major bleeding from venous hypertension of the arterialized vein [7], groin infection and cutaneous scarring [9,10].
Other modalities of treatment that have been described mainly fall into the endovascular group and include covered stent, coil embolization and the utilization of glue. The close proximity of the fistula to the bifurcation of the common femoral artery precluded the use of these modalities in this instance.
Watchful waiting and ultrasound-guided compression have been advocated as first line therapeutic options as they are both non-invasive. However, spontaneous closure is less likely to occur due to the fact that most patients are on anti-platelet and anti-coagulant therapy [11].
Post-catheterization femoral AVFs are not immediately life threatening, but high output cardiac failure, chronic limb oedema and aneurysmal degeneration of the artery are documented consequences [12]. Arteriovenous fistulas are potentially dangerous to patients with cardiac failure.
Risks factors that increase the likelihood of developing AVFs include distal groin punctures, arterial hypertension, female gender, peri-procedural anti-coagulation and anti-platelet therapy [13]. In this instance, the puncture was low at the level of the SFA and vein. It is recommended that ultrasonography is used to direct needle puncture to avoid repeated blind punctures.
Ultrasonography is known to reduce carotid arterial puncture when inserting dialysis catheters in the neck. It is likely that ultrasonography may also assist in the placement of femoral vein catheters by clearly visualizing the common femoral vein as well as the artery and its branches.
Conflict of interest statement. None declared.
References
- 1.Stigall KE, Dorsey JS. Late complications of traumatic arteriovenous fistula. Case report and overview. Ann Surg. 1989;55:180–183. [PubMed] [Google Scholar]
- 2.Katz MD, Hanks SE. Arteriography and transcatheter treatment of extremity trauma. In: Baums, Pentecot MJ, editors. Abrahams’ Angiography, vol III. Philadelphia: Little, Brown and Company; 1997. pp. 857–867. [Google Scholar]
- 3.Cragg AH, Lund G, Rysavy JA, et al. Percutaneous arterial grafting. Radiology. 1984;150:45–49. doi: 10.1148/radiology.150.1.6689786. [DOI] [PubMed] [Google Scholar]
- 4.Mann ML, Veith FJ, Panetta TF, et al. Percutaneous transfemoral insertion of a stented graft to repair a traumatic femoral arteriovenous fistula. J Vasc Surg. 1993;18:299–302. [PubMed] [Google Scholar]
- 5.Straton CS, Tisnado J. Spontaneous arteriovenous fistula of lower extremities: angiographic demonstration in five patients with peripheral vascular disease. Cardiovasc Intervent Radiol. 2000;23:318–321. doi: 10.1007/s002700010079. [DOI] [PubMed] [Google Scholar]
- 6.Beregi JP, Prat A, Willoteaux S, et al. Covered stents in the treatment of peripheral arterial aneurysms: procedural results and midterm follow-up. Cardiovasc Intervent Radiol. 1999;22:13–19. doi: 10.1007/s002709900322. [DOI] [PubMed] [Google Scholar]
- 7.Parodi JC, Schonholz C, Ferreira LM, et al. Endovascular stent graft treatment of traumatic arterial lesions. Ann Vasc Surg. 1999;13:121–129. doi: 10.1007/s100169900230. [DOI] [PubMed] [Google Scholar]
- 8.Linder F. Acquired arteriovenous fistula. Report of 223 operated cases. Ann Chir Gynaecol. 1985;74:1–5. [PubMed] [Google Scholar]
- 9.Muller DWM, Shamir KJ, Ellis SG, et al. Peripheral vascular complications after conventional and complex percutaneous coronary intervention procedures. Am J Cardiol. 1992;69:63–68. doi: 10.1016/0002-9149(92)90677-q. [DOI] [PubMed] [Google Scholar]
- 10.Ruebben A, Tettoni S, Muratore P, et al. Arteriovenous fistulas induced by femoral artery catheterization: percutaneous treatment. Radiology. 1998;209:729–734. doi: 10.1148/radiology.209.3.9844666. [DOI] [PubMed] [Google Scholar]
- 11.Kresowik TF, Khoury MD, Miller BV, et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg. 1991;3:328–336. [PubMed] [Google Scholar]
- 12.Sako Y, Varco RL. Arteriovenous fistula: results of management of congenital and acquired forms, blood flow measurements and observations on proximal arterial degeneration. Surgery. 1970;67:40–61. [PubMed] [Google Scholar]
- 13.Kelm M, Perings SM, Jax T, et al. Incidence and clinical outcome of iatrogenic femoral arteriovenous fistulas: implications of risk stratification and treatment. J Am Coll Cardiol. 2002;40:291–297. doi: 10.1016/s0735-1097(02)01966-6. [DOI] [PubMed] [Google Scholar]