Abstract
Vascular endothelial growth factor (VEGF) is integral to the integrity of the glomerular filtration barrier. Bevacizumab is a humanized monoclonal antibody directed against VEGF with expanding clinical applications for metastatic solid tumours. We describe a case of a 61-year-old female with ovarian cancer and baseline chronic kidney disease who received three doses of bevacizumab and subsequently developed progressive renal clearance dysfunction and nephrotic range proteinuria. A renal biopsy was performed 4 months after drug discontinuation and was consistent with TMA. At baseline, prior to bevacizumab exposure, her estimated glomerular filtration rate (eGFR) was 44 mL/min/1.73 m2 and she had no proteinuria. At the completion of therapy, eGFR was 27 mL/min/1.73 m2 with 1+ proteinuria on urinalysis. Her renal failure and proteinuria continued to progress 5 months after discontinuation of bevacizumab therapy, at which time eGFR was 11 mL/min/1.73 m2 and proteinuria was 5.5 g/24 h. Non-remitting TMA after bevacizumab therapy in patients with pre-existing chronic kidney disease has not been previously reported. Further studies are needed to assess the safety of this drug in patients with chronic kidney disease.
Keywords: bevacizumab, renal failure, thrombotic microangiopathy, VEGF inhibitor
Background
Bevacizumab (Avastin, Genentech, South San Francisco, CA, USA) is a humanized monoclonal antibody directed against vascular endothelial growth factor (VEGF) [1]. The drug's clinical application has expanded from its initial indication for metastatic colorectal cancer to breast, ovarian, renal cell and other solid tumour malignancies [2]. The importance of VEGF to the integrity of the glomerular filtration barrier is evidenced by the induction of proteinuria with anti-VEGF therapy, which, along with hypertension, may occur in over 50% of treated patients [3]. In addition, nine cases have been reported of biopsy-proven, renal-limited thrombotic microangiopathy (RL-TMA) after exposure to bevacizumab [4–7]. In all of these cases, the clinical findings of renal dysfunction and proteinuria improved or resolved after cessation of bevacizumab.
We report the first case of a patient with baseline chronic kidney disease and advanced ovarian cancer who received bevacizumab therapy and developed progressive RL-TMA marked by renal function decline and nephrotic range proteinuria despite cessation of anti-VEGF therapy.
Case
The patient is a 61-year-old female with a medical history significant for metastatic ovarian cancer. She was initially diagnosed in August 2004 and treated with carboplatin and paclitaxel. In September 2005, she was treated with doxorubicin and bevacizumab for relapse and attained remission. In mid-2007, she developed a progressive rise in serum CA-125 along with increasing pelvic and abdominal adenopathy. In August 2007, treatment with doxorubicin was initiated and, in January 2008, bevacizumab therapy was re-initiated in combination with the doxarubicin. She received six total infusions of bevacizumab (15 mg/kg/dose) between January and March 2008. One week prior to starting this regimen, she was normotensive, had no proteinuria by urine dipstick and serum creatinine was 1.7 mg/dL.
In February 2008, after the second dose of bevacizumab, she developed marked hypertension (blood pressure 179/ 89 mmHg). Her serum creatinine at that time was 2.5 mg/dL. A renal ultrasound showed preserved renal cortical thickness (1.2 cm or greater bilaterally) and renal sizes (left and right kidneys each >12 cm in long axis). She received her last dose of bevacizumab in mid-March and her serum creatinine was 2.6 mg/dL with 70–100 mg/dL proteinuria by urine dipstick.
She presented to our institution in early April 2008. Additional medical history was notable only for a remote history of isolated nephrolithiasis. Medications included nifedipine and metoprolol. Her examination was significant for a blood pressure of 186/98, and 2+ lower extremity pitting oedema. Initial lab work showed a serum creatinine of 2.1 mg/dL and an estimated 2.0 g of proteinuria/24 h by random urine protein-to-urine creatinine ratio. There was no evidence of anaemia or thrombocytopaenia on admission. Urinalysis revealed 3+ protein by urine dipstick and no blood. Examination of the urine sediment showed two to four fragmented granular casts/hpf and two to four hyaline casts/hpf.
Over the ensuing 3 months, serial changes to her antihypertensive medication regimen were made in an attempt to control her blood pressure prior to the renal biopsy. Her renal function continued to decline in conjunction with increased proteinuria. In July 2008, her serum creatinine was 3.6 mg/dL, and a random urine protein-to-creatinine ratio was 5.5 g/g. A renal biopsy was performed. Notable histologic findings include widened and irregular capillary loops, probable thrombi in peripheral capillary loops and areas of mesangiolysis with extravasated red blood cells consistent with thrombotic microangiopathy. In addition, the surrounding renal parenchyma demonstrated patchy interstitial fibrosis and tubular atrophy with associated chronic interstitial inflammation (Figure 1a and b).
Fig. 1.
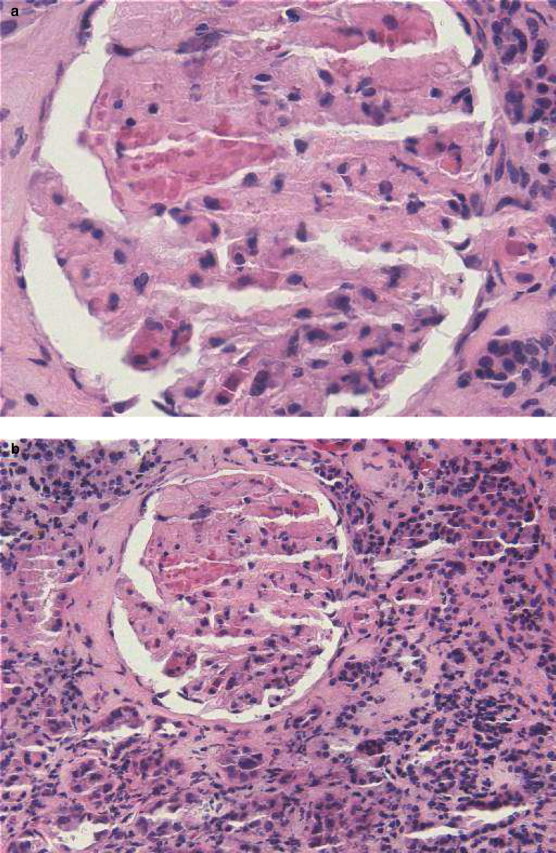
(a) Glomerulus showing mesangiolysis with extravasated RBCs and probable thrombus in peripheral capillary loop (haematoxylin and eosin, 400×). (b) Renal parenchyma displaying interstitial fibrosis and tubular atrophy with associated chronic inflammation (haematoxylin and eosin, 200×).
After discussions regarding prognosis and treatment options with her and her primary oncologist, she declined further therapy and began hospice care in September 2008 with a serum creatinine of 4.4 mg/dL (eGFR 11 mL/min/ 1.73 m2). This occurred ∼6 months after her last exposure to bevacizumab.
Discussion
We describe a patient with chronic kidney disease prior to the re-initiation of bevacizumab who developed progressive renal failure secondary to RL-TMA. To our knowledge, this is the first report of VEGF inhibitor-associated TMA that failed to resolve after withdrawal of the drug.
Since the original report by Frangié et al., in 2007, eight additional cases of bevacizumab-associated renal TMA have been reported. All nine of these patients had normal renal function prior to initiation of anti-VEGF therapy with the exception of one patient, a 70-year-old male with a single kidney after nephrectomy for renal cell carcinoma, who had a baseline eGFR of 41 mL/min. After drug withdrawal, two patients died. Proteinuria improved in the remaining seven patients. Time course to resolution of proteinuria was only reported in five of these cases (median 3 months, range 2–9.5 months). Renal clearance function after drug withdrawal was reported in four of the nine cases, and improved to baseline in three and stabilized at 1.4 mg/dL in one. This occurred within the same time frame as proteinuria resolution. Consistent with these observations, most authors reporting on this condition note that anti-VEGF inhibitor TMA is a reversible disease process after the drug is withdrawn. This contrasts with our experience (Figure 2).
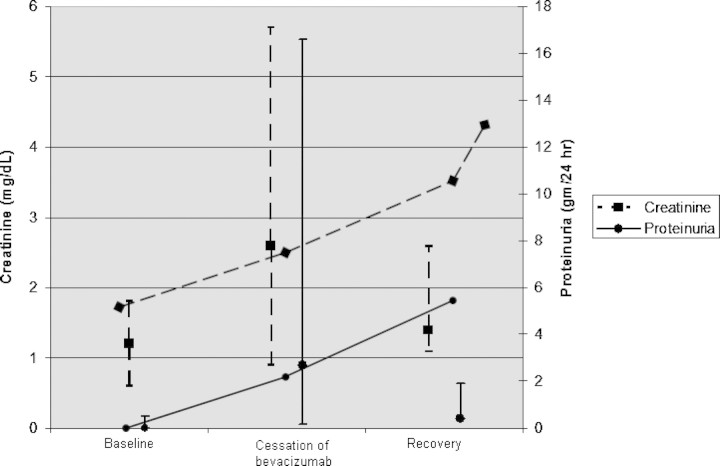
Fig. 2.
Serum creatinine and proteinuria in patients before, during and after bevacizumab therapy. Baseline: median and range of baseline serum creatinine (n = 8) and proteinuria (n = 8) for patients prior to with bevacizumab therapy. Cessation of bevacizumab: median and range of peak serum creatinine (n = 7) and proteinuria (n = 9) for patients during bevacizumab therapy. Recovery: median and range of recovery serum creatinine (n = 5) and proteinuria (n = 7) for patients after recovery with bevacizumab therapy. For previous cases, if a serum creatinine value was not reported but kidney function was described as ‘normal’, a serum creatinine value of 1.2 was assigned. Vertical lines are median and range values for serum creatinine (dashed) and proteinuria (solid) in previously reported cases; the horizontal lines are values for creatinine and proteinuria for the patient reported in this case.
The reasons behind this patient's lack of response to drug withdrawal are not clear, but may relate to her pre-existing chronic kidney disease, co-existent severe hypertension and/or heavy proteinuria. Additionally, we do not know what role, if any, the prior bevacizumab exposure played in this disease process. We do not have information beyond measurement of serum creatinine and urinalysis about the condition of her kidneys prior to re-exposure to the drug in early 2008. Although the patient had stable chronic kidney disease with no proteinuria prior to re-exposure, without a previous biopsy we do not know the severity of her underlying kidney disease and how this may have impacted her inability to tolerate bevacizumab therapy. However, from the biopsy performed ∼4.5 months after stopping the VEGF inhibitor, we do know that the TMA was still active, and evidence for an ongoing and active glomerular lesion is clinically supported by worsening nephrotic-range proteinuria at the time of, and after, renal biopsy.
Our case raises several important questions. What is the natural history of VEGF inhibitor-associated RL-TMA in patients with pre-existing chronic kidney disease? Is the disease course different than that in patients with normal baseline renal function? What is the appropriate diagnostic and management strategy for patients with chronic kidney disease exposed to VEGF-inhibitor therapy who develop proteinuria, hypertension and/or renal failure? The answers to these questions require additional data. While the indication for therapeutic plasma exchange for cases of drug-associated TMA is not clear, it is not at all apparent that this intervention would have yielded substantial return of renal clearance function in this patient with fairly advanced tubulointerstitial fibrosis [8].
Currently, neither the drug manufacturer nor the FDA provides any guidance regarding use of this class of medication in patients with pre-existing kidney disease [9]. Given our reported findings, we believe it prudent to consider additional counselling of patients with chronic kidney disease prior to initiating anti-VEGF therapy about the risks of TMA associated with this drug and the lack of prognostic, patient-level data which would allow for an accurate estimate of these risks. Additionally, given that patients developing VEGF inhibitor-associated TMA do not universally exhibit the clinical, multi-system organ manifestations and laboratory findings classically associated with TMA, we recommend a low threshold for renal biopsy in patients administered these agents who develop proteinuria, hypertension and renal insufficiency.
Conflict of interest statement. The authors have no financial or other conflicts of interest related to the work presented.
References
- 1.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 2.Midgley R, Kerr D. Bevacizumab—current status and future directions. Ann Oncol. 2005;16:999–1004. doi: 10.1093/annonc/mdi208. [DOI] [PubMed] [Google Scholar]
- 3.Izzedine H, Rixe O, Billemont B, et al. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50:203–218. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Frangié C, Lefaucheur C, Jacquot C, et al. Renal thrombotic microangiopathy caused by anti-VEGF antibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 2007;8:177–178. doi: 10.1016/S1470-2045(07)70037-2. [DOI] [PubMed] [Google Scholar]
- 5.Roncone D, Satoskar A, Nadasdy T, et al. Proteinuria in a patient receiving anti-VEGF therapy for metastatic renal cell carcinoma. Clin Pract Nephrol. 2007;3:287–293. doi: 10.1038/ncpneph0476. [DOI] [PubMed] [Google Scholar]
- 6.Izzedine H, Brocheriou I, Deray G, et al. Thrombotic microangiopathy and anti-VEGF agents. Nephrol Dial Transplant. 2007;22:1481–1482. doi: 10.1093/ndt/gfl565. [DOI] [PubMed] [Google Scholar]
- 7.Eremina V, Jefferson J, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remuzzi G, Ruggenenti P. The hemolytic uremic syndrome. Kidney Int. 1995;48:2–19. doi: 10.1038/ki.1995.261. [DOI] [PubMed] [Google Scholar]
- 9.Avastin (bevacizumab) labeling information. http://www.fda.gov/cder/foi/label/2008/125085s91lbl.pdf.Accessed 10/28/2008 . [Google Scholar]