Abstract
The second part of this in-depth clinical review focuses on drugs used in the prevention of AKI in the patient at risk and/or in the management of the patient with incipient AKI. Among the drugs used to maintain a normal renal perfusion pressure, norepinephrine and vasopressin are most commonly used in hypotensive critically ill patients. The most recent RCT did not find a difference between low-dose vasopressin plus norepinephrine and norepinephrine alone in patients with septic shock, suggesting that either approach is reasonable. However, vasopressin may be beneficial in the less severe septic shock subgroup. Loop diuretics may convert an oliguric into a non-oliguric form of AKI that may allow easier fluid and/or nutritional support of the patient. Volume overload in AKI patients is common and diuretics may provide symptomatic benefit in that situation. However, loop diuretics are neither associated with improved survival, nor with better recovery of renal function in AKI. Among the renal vasodilating drugs, the routine administration of dopamine to patients for the prevention of AKI or incipient AKI is no longer justified. On the other hand, although additional studies may be warranted, fenoldopam may appear to be a likely candidate for the prevention of AKI, particularly in critically ill patients, if the positive results obtained in some recent studies are confirmed. Trials with natriuretic peptides were in general inconclusive but despite the fact that nesiritide is currently approved by the FDA only for the treatment of heart failure, this vasodilator may in the future play a role in the prevention of AKI, particularly in association with heart failure and cardiac surgery. The most recent trials seem to confirm a potential positive preventive effect of N-acetylcysteine (NAC), particularly in contrast-induced nephropathy (CIN), NAC alone should never take the place of IV hydration in patients at risk for CIN; fluids likely have a more substantiated benefit. At present, initiation of statin therapy for the prevention of CIN cannot be recommended, but these drugs should not be stopped before a radiological intervention in patients on chronic statin therapy. Rasburicase is very effective in the prevention of acute tumour lysis syndrome. Erythropoietin (EPO) has tissue-protective effects and prevents tissue damage during ischaemia and inflammation, and currently trials are performed with EPO in the prevention of AKI post-cardiac surgery, CIN and post-kidney transplantation. From this review it becomes clear that single-drug therapy will probably never be effective in the prevention of AKI and that multiple agents may be needed to improve outcomes. In addition, drugs should be administered early during the course of the disease.
Keywords: acute kidney injury (AKI), diuretics, drug prevention, N-acetylcysteine, statins, vasodilators, vasopressors
Maintaining renal perfusion pressure with vasoactive drugs
An excellent review on the different vasoactive drugs aimed at maintaining renal perfusion pressure has recently been published [1].
In the acute setting, the two most significant threats to renal perfusion pressure are systemic arterial hypotension and increased intra-abdominal pressure (including so-called abdominal compartment syndrome). Given the rather scarce available evidence, specific recommendations to maintain renal perfusion are difficult to make. The following general recommendations apply. First, vasopressor medications (e.g. norepinephrine) should be used only to treat arterial hypotension once intravascular volume has been restored. In practice, vasopressors are often started as volume loading is underway and discontinued if no longer required, once hypovolaemia has been reversed [2].
Secondly, there is no evidence from clinical studies or appropriately designed animal experiments [3,4] that norepinephrine is associated with increased risk of AKI when used to treat arterial hypotension. Indeed, a large observational study [5] and small RCTs [6,7] suggest that other vasopressors, like dopamine, may be less efficacious and possibly associated with lower survival. Thirdly, specific arterial pressure targets for titration of therapy to avoid renal hypoperfusion are not known. Many clinicians and clinical protocols target a mean arterial pressure of 60–65 mmHg. However, patients with long-standing hypertension and/or renal vascular disease may require substantially higher pressures to maintain renal perfusion. Indeed, in many hospital patients, including the setting of critical illness, absolute hypotension (defined as a systolic blood pressure <90 mmHg) has been shown to be associated with the development of AKI [8–20]. However, in many patients with AKI such an episode of absolute hypotension is absent or at least undetected and this form of AKI has been called ‘normotensive acute renal failure’ [21]. A recent study in mainly elderly, non-critically ill ward patients but with multiple co-morbidities, found that there is still an association between blood pressure and AKI [22]. A decrease in systolic blood pressure relative to a pre-morbid value (so-called relative systolic hypotension) was a significant independent predictor of the development of AKI. Whether therapeutically induced return of these patients’ blood pressure to pre-morbid levels may be beneficial is speculative but the ‘usual’ targets for blood pressure treatment in patients with AKI should probably be more focusing on correction of relative hypotension than on absolute blood pressure values.
Fourthly, intra-abdominal hypertension is associated with decreased renal perfusion and may result in AKI [23–25]. Prompt recognition, often guided by urinary bladder pressure measurement, and surgical treatment offer the best potential for recovery [23–25].
Recent guidelines on the management of septic shock [26] have advocated the use of aggressive fluid resuscitation and, if hypotension persists, administration of either norepinephrine or dopamine. However, catecholamines such as norepinephrine and dopamine have adverse effects and may occasionally induce mortality. Vasopressin levels are reduced during septic shock, and exogenous administration of vasopressin has been associated with potent vasopressor effects in several observational studies (for review, see [27]). A recent well-conducted, randomized, multicentre, controlled trial (VASST) involving 778 patients with septic shock [28] evaluated low-dose vasopressin (0.01–0.03 U/min) added to norepinephrine as compared with norepinephrine alone. No difference between the vasopressin and norepinephrine groups in the primary end point of 28-day or 90-day mortality was found. The study further suggested that patients with less severe septic shock (those with a requirement for 5–14 μg of norepinephrine per minute at baseline) had a significant reduction in mortality with vasopressin therapy. It is of note that the overall incidence of new cases of renal failure was similar in both treatment groups (66.5% versus 67.5%). However, in the patients who were in the RIFLE ‘at-risk’ class, the vasopressin treated patients (n: 53) compared to the norepinephrine patients (n: 53) had significantly reduced 28-day and 90-day mortality and these differences remained significant after adjustment for potential confounders. The at-risk patients treated with vasopressin were also less likely to develop renal failure over the 28-day study period compared to the at-risk patients treated with norepinephrine (21.2% versus 41.2%, P < 0.02) [29]. Thus, the VASST did not find a difference between low-dose vasopressin plus norepinephrine and norepinephrine alone suggesting that either approach is reasonable. However, vasopressin may be beneficial in the less severe septic shock subgroup (those patients on <15 μg/min norepinephrine at baseline) and possibly for patients at risk for renal failure. As described above, in part 1 the timing of vasopressor and other therapy, rather than the specific agent, is more decisive in this situation.
Loop diuretics
Oliguria is generally recognized as a bad prognostic sign in patients with incipient or established AKI [12,30,31]. The temptation to increase urine output in patients with or at risk for AKI is therefore great. A recent observational study in the USA reported that 59% of the patients with ARF received diuretics at the time of nephrology consultation [32]. A multinational, multicentre survey of intensivists and nephrologists [33] revealed that the use of furosemide was most common (67.1%), was delivered primarily intravenously (71.9%) and by bolus dosing (43.3%). Pulmonary oedema was a prime indication for diuretic use (86.3%). Similarly, in a small single-centre prospective evaluation of patients with sepsis-induced AKI, Van Biesen et al. [34] found that 72% had received diuretic therapy, mostly with furosemide. Clearly, most ICU clinicians are familiar with the administration, pharmacology and adverse effects of furosemide [33]. Despite this ubiquitous use, it is not clear whether loop diuretics, besides increasing urine output, have also a beneficial effect on renal function in AKI. Two recent meta-analyses [35,36] and one recent review [37] concluded that loop diuretics were neither associated with improved survival benefit in AKI nor with better recovery of renal function despite reduction in the oliguric period. Furthermore, Mehta et al. [32] found that diuretic use was associated with significantly increased risk of death or non-recovery of renal function. Most of the increased risk was seen in patients unresponsive to high doses of diuretics, implying they had more severe disease [38]. A multinational, multi-centre, observational study [39] found that although diuretic use was not significantly associated with increased mortality, there was no evidence of benefit either. Loop diuretics may, however, convert an oliguric into a non-oliguric form of AKI that may allow easier fluid and/or nutritional support of the patient. Volume overload in AKI patients is common and diuretics may provide symptomatic benefit in that situation.
Loop diuretics in the setting of impaired renal function are not without hazards. Transient episodes of tinnitus and/or vertigo and very rarely deafness may be present if high doses are administered intravenously in <6 h. Furosemide dosage should not exceed 1000 mg/day. Co-prescription of aminoglycosides with diuretics increases the risk of ototoxicity and should be avoided.
Based on these data, it can be concluded that there is no evidence to support the use of loop diuretics in the prevention of ARF.
Mannitol
As discussed above, mannitol has potential beneficial effects in rhabdomyolysis by stimulation of the diuresis and lowering the intracompartimental pressure in the affected crushed limbs [40–42].
A second situation where mannitol can be considered is perioperative renal protection particularly in cardiovascular surgery, where it is often added to the cardiopulmonary bypass pump prime to reduce the incidence of renal dysfunction; however, the studies are not very convincing [43]. A recent double-blind RCT in cardiac surgical patients with pre-operative SCr < 130 μmol/L found, however, no differences between the mannitol and control patients for any measured variable of renal function [44].
Several small trials have investigated the effect of mannitol added to fluids in the prevention of CIN. In one trial, 78 patients with stable chronic renal failure about to undergo coronary angiography were randomly assigned to one of three regimens [45]. One of the regimens added mannitol to half-isotonic saline. The incidence of AKI (defined as a rise in the SCr of at least 0.5 mg/dL) was lowest in the group treated with saline alone, and mannitol was of no added benefit.
In another study [46], patients were randomly assigned to receive saline or one of three renal vasodilator/diuretic drugs [dopamine (2 μg/kg/min), mannitol (15 g/dL in a one-half isotonic saline solution given at 100 mL/h) or atrial natriuretic peptide (ANP)]. Dopamine, mannitol and atrial natriuretic peptide were associated with a much higher incidence of renal dysfunction in diabetic subjects.
The only setting, besides rhabdomyolysis and post-cardiovascular surgery, where mannitol is probably protective is renal transplantation [47–49]. The sparse controlled data available have shown that 250 mL of mannitol (20%) given immediately before vessel clamp removal reduces the incidence of post-transplant AKI, as indicated by a lower requirement of post-transplant dialysis. However, 3 months after transplantation no difference is found in kidney function compared with patients who did not receive mannitol [50].
Some precautions should be taken when mannitol is used, including careful monitoring of the urinary losses because mannitol can lead to both volume depletion and hypernatraemia. Mannitol administered in very high doses, or to patients with reduced renal excretion, can cause hyperosmolality, volume expansion and hyperkalaemia because of passive movement of potassium out of the cell. AKI may occur if patients are treated with >200 g of mannitol per day [51]. Mannitol should be discontinued if the osmolal gap during therapy rises above 55 mosmol/kg and when no diuretic response occurs.
Renal vasodilators
Dopamine
In experimental animals and healthy human volunteers, renal dose dopamine (<5 μg/kg of body weight/min) increases RBF and, to a lesser extent, GFR. According to several meta-analyses dopamine was unable to prevent or alter the course of ischaemic or nephrotoxic AKI [52–58]. Furthermore, dopamine, even at low doses, can induce tachy-arrhythmia’s, myocardial ischaemia, and extravasation out of the vein can cause severe necrosis [57]. Thus, the routine administration of dopamine to patients for the prevention of AKI or incipient AKI is no longer justified.
Fenoldopam
Fenoldopam is a highly selective dopamine type 1 agonist that preferentially dilates the renal and splanchnic vasculature. Many, but often underpowered clinical trials have shown inconsistent results, potentially related to the fact that fenoldopam can cause a dose-dependent hypotension and can thus aggravate rather than protect the kidney by decreasing renal perfusion pressure [59]. A recent systematic review [60] of 13 studies in patients undergoing cardiovascular surgery showed that fenoldopam significantly reduced the need for RRT (odds ratio = 0.37) and in-hospital death (odds ratio = 0.46). The dose of fenoldopam in these studies ranged from 0.03 to 0.1 μg/kg/min and was administered between 2 and 72 h of surgery. This meta-analysis besides containing a heterogeneous mix of abstracts, and randomized and non-randomized studies included a number of recent single-centre studies [61,62]; a correct interpretation of all these studies becomes difficult. One prospective, multiple-centre RCT in critically ill patients with incipient renal dysfunction randomized patients to receive 2 μ/kg/min dopamine or 0.1 μg/kg/min fenoldopam mesylate as continuous infusion over a 4-day period [63]. Fenoldopam produced a more significant reduction in creatinine values compared with dopamine after 2, 3 and 4 days of infusion and the maximum decrease in creatinine compared with baseline was significantly greater in the fenoldopamp group. This study thus suggests that in the setting of acute early renal dysfunction, and before severe renal failure occurs, the attempt to reverse renal hypoperfusion with fenoldopam is more effective than with low-dose dopamine.
A single-centre study [64] using a longer duration of fenoldopam (mean, 10 days) at a dose of 0.09 μg/kg/min in critically ill septic patients also showed a reduction in AKI.
Fenoldopam has failed to prevent CIN in patients with chronic renal insufficiency. In a large RCT, patients with creatinine clearance <60 mL/min were randomized to fenoldopam or placebo [65]. The primary end point of CIN (25% increase in SCr within 96 h after the procedure) occurred equally in both groups.
Overall, it can be concluded that additional studies may be warranted and, if the positive results obtained in some recent studies are confirmed, fenoldopam may appear to be a likely candidate for the prevention of AKI, particularly in critically ill patients.
Natriuretic peptides
Natriuretic peptides are hormones secreted by the heart in response to volume overload with increased cardiac stretch and other stimuli. ANP is a 28-amino acid peptide synthesized by atrial myocytes. Brain natriuretic peptide (BNP) is a 32-amino acid peptide synthesized in the brain and in the heart.
ANP and BNP are systemic and renal vasodilators; they inhibit renal tubular sodium reabsorption, attenuate the activation of the renin–angiotensin–aldosterone system and lower the oxygen requirements in several nephron segments (for recent overviews and reviews, see [66–69]).
Synthetic analogues of ANP have shown promise in the management of AKI in the laboratory setting. To date, this promise has failed to translate into clinically apparent benefit, and a large multicentre, prospective RCT of anaritide, a synthetic analogue of ANP, could not show clinically significant improvement in dialysis-free survival or overall mortality in patients with AKI [70].
Ularitide (urodilantin) is a natriuretic pro-ANP fragment produced within the kidney. In a small randomized trial, ularitide did not reduce the need for dialysis in patients with AKI [71].
Interestingly, a small, single-centre RCT [72] studied 61 patients after cardiac surgery using a continuous infusion of low-dose (50 ng/kg/min) human recombinant ANP (rhANP). This trial, unlike the larger studies in the past, showed a decreased use of dialysis and improved dialysis-free survival in treated patients compared to placebo. Although the results of this small study are interesting, further larger RCTs are necessary in the cardiac surgical population with low-dose rhANP before recommending it for routine clinical use.
Recombinant BNP (nesiritide) has the same amino acid sequence as human B-type natriuretic peptide (BNP), which is secreted by the ventricles in response to myocardial stretch and is approved for treatment of symptomatic acute decompensated heart failure. The role of nesiritide in patients with left ventricular dysfunction (ejection frac- tion ≤40%) undergoing coronary artery bypass grafting (CABG) using CPB was recently determined [73]. The drug was administered as a 24- to 96-h infusion of 0.01 μg/kg/min. Compared with placebo, nesiritide was associated with a significantly attenuated peak increase in SCr and a smaller fall in eGFR during hospital stay or by study Day 14, and a greater urine output during the initial 24 h after surgery. In addition, nesiritide-treated patients had a shorter hospital stay and lower 180-day mortality. Although SCr increased in both groups, it returned to baseline within 12 h in those treated with nesiritide and remained elevated in the placebo group throughout hospitalization. Renal protection was greatest in patients with pre-existing renal dysfunction. Another recent RCT [74] evaluated the impact of nesiritide on renal function in patients with acute decompensated heart failure and baseline renal dysfunction. Subjects received either nesiritide (0.01 μg/kg/min with or without a 2 μg/kg bolus) or placebo (5% dextrose in water) for 48 h in addition to their usual care. Both groups had similar baseline parameters like age, blood pressure and SCr (1.82 versus 1.86 mg/dL). There were no significant differences in the incidence of a 20% creatinine rise or in the change in SCr. There were no significant differences in the secondary end points of change in weight, dose of intravenous furosemide administered, discontinuation of the infusion of nesiritide due to hypotension or 30-day death/hospital readmission.
Although currently approved by the FDA only for the treatment of heart failure, nesiritide may in the future also play a role in the prevention of AKI in heart failure and cardiac surgery.
Adenosine antagonists (theophylline)
Animal studies using theophylline pretreatment have demonstrated attenuation of the intrarenal vasoconstriction after the administration of radiocontrast media. The acute reduction in GFR induced may therefore be theoretically minimized or prevented in some patients by theophylline or aminophylline (presumably via inhibition of the effect of adenosine). A 2005 meta-analysis of nine controlled trials of 585 patients [75] (theophylline versus controls) concluded that theophylline may reduce the incidence of CIN although the absolute benefit was small and patients studied were at relatively low risk (only one case required dialysis). This meta-analysis found that findings are inconsistent across studies. In contrast, concurrent administration of the anti-platelet agent dipyridamole may increase contrast toxicity by enhancing the action of adenosine [76]. There is at present little evidence to recommend theophylline for the prevention of CIN.
Other vasoactive drugs
It is actually unclear if pharmacologic inhibition of renal vasoconstriction with endothelin receptor blockers, nifedipine, captopril and prostaglandin E protects patients at high risk for developing AKI after contrast administration.
For example, a multicentre, double-blind randomized trial [77] evaluating a non-specific endothelin receptor blocker in high-risk patients undergoing coronary angiography showed that compared with placebo, a significantly higher percentage of patients who received active therapy sustained CIN (56 versus 29%).
N-Acetylcysteine (NAC)
There is evidence that an increased renal free-radical production may in part be responsible for the renal injury in both post-ischaemic and nephrotoxic AKI. For reviews see [78–83]. NAC, a thiol-containing antioxidant, ameliorates ischaemic renal failure in animals [84] and has been used to prevent AKI in patients with acetaminophen-induced liver failure [85]. Based on these observations, several clinical studies evaluated the efficacy of NAC, mainly in the prevention of CIN and post-cardiac surgery AKI.
There exist great heterogeneity and conflicting results in the available clinical trials and meta-analyses examining the effectiveness of NAC in the prevention of CIN.
Before discussing the role of NAC in the prevention of CIN, it is appropriate to briefly remind the problems associated with the definition of CIN. CIN is typically defined in the recent literature as an increase in SCr occurring within the first 24 h after contrast exposure and peaking up to 5 days afterwards. In most instances, the rise in SCr is expressed either in absolute terms (0.5–1.0 mg/dL) or as a proportional rise in SCr of 25 or 50% above the baseline value. The most commonly used definition in clinical trials is a rise in SCr of 0.5 mg/dL or a 25% increase from the baseline value, assessed at 48 h after the procedure [86]. Most studies of contrast-induced nephropathy lack controls to distinguish it from nephropathy due to other causes. A recent study assessed the frequency and magnitude of SCr changes over 5 days in hospitalized patients not receiving iodinated contrast material to compare with SCr changes in publications regarding CIN [87]. Fluctuations in SCr occurred frequently but among the 32 161 patients, more than half showed a change of at least 25% and more than two-fifths, a change of at least 0.4 mg/dL and these increases in SCr were not different from the incidences of CIN previously published in the literature. These results not only suggest that the risk of contrast material in inducing nephropathy may have been overestimated in many studies in hospitalized patients but that prevention studies in CIN, relying on small changes in SCr must be interpreted with caution.
After publication of the seminal study by Tepel et al. [88], where NAC (600 mg orally twice daily) showed some protection, a large number of studies, mostly with relatively small sample sizes, have been published. The results have been remarkably varied with some studies finding great efficacy with NAC but many others finding no significant benefit [89]. A recent, large, single-centre RCT [90] not only found a clear protecting but also a dose- dependent effect of NAC in CIN.
Subsequently, several meta-analyses have pooled the existing data and have consistently found that NAC along with hydration decreases incidence of CIN compared to hydration alone [91–94] but with a significant heterogeneity in NAC effect across studies. A very recent meta-analysis [95] on the efficiency of drug prevention in CIN included all RCTs that administered NAC, theophylline, fenoldopam, dopamine, iloprost, statin, furosemide or mannitol to a treatment group. In the 41 studies included, NAC reduced the risk for CIN more than saline infusion alone, whereas furosemide increased it. All the remaining agents did not significantly affect risk. It was concluded that NAC is more renoprotective than hydration alone. This meta-analysis, together with another recent in depth review on CIN [96], thus suggests that the administration of NAC, together with fluid loading may be useful, particularly in high-risk patients.
It has been suggested that NAC may decrease SCr without affecting GFR [97] by activating creatinine kinase [98] and possibly by increasing the tubular secretion of creatinine. However, a more recent study in which a ‘double-dose’ NAC was administered in the absence of iodinated contrast media to patients with stable CKD showed no effect of NAC on either serum creatinine or cystatin C levels [99].
The screening and emergency preventative measures against CIN in critically ill patients, where the ‘routine hydration’ and oral NAC administration cannot be applied, raise a number of difficult problems [100]. In these patients, a modified preventative protocol should be applied. An analysis [101] of seven randomized trials where prevention was applied within 2 h pre-contrast (NAC in three, theophylline in two and bicarbonate and ascorbic acid in one trial) revealed that interventions (theophylline, bicarbonate and ascorbic acid) were appropriate to an emergency department setting and decreased the risk of CIN. In this analysis, the case for the effectiveness of NAC was less certain. In this regard, a prospective multicentre RCT was conducted [102] to test the hypothesis that a rapid protocol of an intravenous dose of NAC [150 mg/kg in 500 mL saline (0.9%)] over 30 min immediately before contrast exposure and followed by 50 mg/kg in 500 mL saline (0.9%) over the subsequent 4 h would be more effective at inhibiting CIN in high-risk patients than prolonged saline hydration alone. In the control group, saline was given at a rate of 1 mL/kg/h for 12 h pre- and post-procedure. CIN occurred significantly less frequent in the NAC group compared to the hydration group.
Although the most recent literature recommends the use of NAC in high-risk patients for CIN given its potential benefit, low cost and excellent side-effect profile, it should never take the place of IV fluids, which likely have a more substantial benefit.
NAC has also been investigated in the prevention of post-cardiac surgery AKI [103]. Patients with CKD undergoing heart surgery were randomized to either NAC 600 mg orally twice daily or placebo for a total of 14 doses (three preoperative doses). This study showed that prophylactic perioperative NAC administration does not seem to prevent AKI after cardiac surgery.
Statins
Pleiotropic effects of statins include improvement of endothelial dysfunction, increased nitric oxide bioavailability, antioxidant properties, inhibition of inflammatory responses and stabilization of atherosclerotic plaques. These and several other emergent properties could act in concert with the potent low-density lipoprotein cholesterol-lowering effects of statins to exert early as well as lasting cardiovascular protective and potentially reno-protective effects [104].
A large retrospective study demonstrated that patients who continued on statins during cardiovascular procedures including percutaneous coronary intervention (PCI) and CAB grafting have lower rates of AKI [105]. A recent prospective study [106] evaluated the influence of potential benefit of pre-procedural statins on CIN and on the long-term (4-year) cardiovascular outcome. CIN was defined as a post-procedural increase in SCr of ≥0.5 mg/dL or >25% from baseline. Statin-treated patients had a significantly lower incidence of CIN (3% versus 27%, P < 0.0001). No benefit was observed in patients with a pre-existing creatinine clearance <40 mL/min. During the follow-up, CIN was a predictor of poorer cardiovascular outcome, a well-known observation of CIN [96]. These results may lend further support to utilization of statins as adjuvant pharmacologic therapy before PCI.
In contrast, another recent propensity-based analysis [107] showed that neither the presence nor timing of perioperative statin therapy was associated with improved renal outcomes in patients undergoing a range of major vascular procedures. A possible exception is early postoperative re-initiation of statin therapy in chronic statin users. All these discrepant results of the available literature preclude a definitive statement on the use of statin therapy as a means of preventing postoperative renal dysfunction. At present, initiation of statin therapy for the prevention of CIN cannot be recommended, but these drugs should not be stopped before a radiological intervention in patients on chronic therapy.
Ascorbic acid
Ascorbic acid has been tested in a multicentre, blinded, placebo-controlled trial and been shown to reduce rates of CIN. The dose of ascorbic acid (vitamin C over the counter) used in this trial was 3 g orally the night before and 2 g orally twice a day after the procedure [108]. However, a second large well-designed trial (the REMEDIAL trial mentioned above) [109] found that ascorbic acid did not provide added benefit to a prophylactic regimen of isotonic saline plus NAC among patients at high risk for CIN. In summary, data are insufficient to support the use of ascorbic acid for the prevention of CIN.
Prophylactic dialysis in CIN
It is known that contrast media can be effectively removed from the blood of patients with chronic renal failure by haemodialysis. Marenzi et al. [110] compared haemofiltration, with isotonic-saline hydration, in preventing CIN in patients with renal failure, who were undergoing coronary interventions. Haemofiltration (fluid replacement rate, 1000 mL/h without weight loss) and saline hydration were initiated 4–8 h before the coronary intervention and were continued for 18–24 h after the procedure was completed. An increase in the SCr concentration of >25% from baseline after the coronary intervention occurred less frequently among the patients in the haemofiltration group compared to control patients (5% versus 50%). Temporary RRT was required in 25% of the control patients and in only 3% of the patients in the haemofiltration group. In-hospital mortality was 2% in the haemofiltration group and 14% in the control group while the cumulative 1-year mortality was 10% and 30%, respectively. A subsequent study by the same authors confirmed these beneficial effects but suggested that, not unexpectedly, the best results are obtained by pre-haemofiltration [111].
Of course, continuous haemofiltration is a rather costly and time-consuming procedure, which is not available in every hospital. In this respect, intermittent haemodialysis is simpler, less expensive and more available, but previous studies on the prophylactic use of haemodialysis in CIN showed controversial results.
Lee et al. [112] randomly assigned 82 patients with chronic renal failure (residual creatinine clearance <25 mL/min), and referred for coronary angiography, to receive either normal saline IV and prophylactic haemodialysis or fluid supplements only. Haemodialysis was performed with a polysulfone high flux dialysis membrane. Compared with the dialysis group, the SCr concentrations in the control group were significantly higher at Day 4 and at peak levels. Temporary RRT was required in 35% of the control patients and in only 2% of the dialysis group. Thirteen percent of the control patients, but none of the dialysis patients required long-term dialysis after discharge. It was concluded that prophylactic haemodialysis is effective in improving renal outcome in these high-risk patients undergoing coronary angiography.
However, recent systematic reviews and meta-analysis of several studies [113,114], including the one by Marenzi et al. [110], found that peri-procedural extracorporeal blood purification (haemodialysis or continous RRT) does not reduce the incidence of CIN compared to standard medical prevention and cannot at present be recommended.
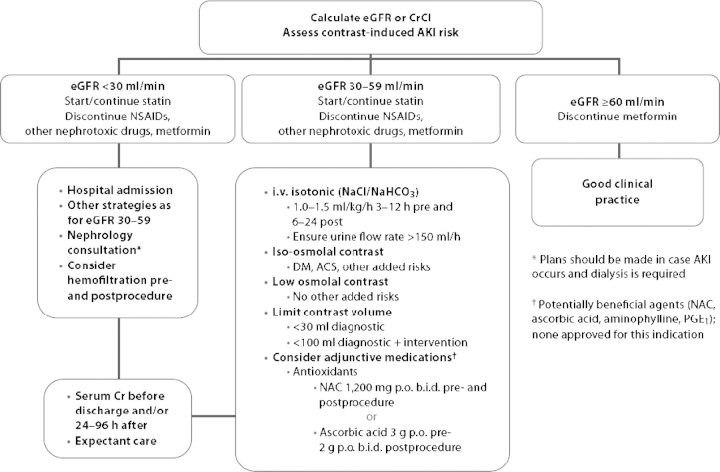
A practical algorithm summarizing the clinical approach and possible preventative measures to be applied in patients with progressively lower pre-contrast GFR is depicted in Figure 1 taken from [86].
Fig. 1.
Advanced algorithm for the management of patients receiving iodinated contrast media (taken from reference 86 after permission). ACS: acute coronary syndromes; NAC: N-acetylcysteine; NSAIDs: non-steroidal anti-inflammatory drugs.
Anti-tumour lysis syndrome agents
Allopurinol and the recently introduced rasburicase, a recombinant urate oxidase preparation, decrease the synthesis of uric acid in patients with rapidly growing tumours like leukaemia and lymphoma and are prone to uric-acid nephropathy and tumour lysis syndrome [115]. Rasburicase is very effective in the prevention of this complication [115]. The FDA-recommended dosing guidelines for rasburicase in paediatric patients are 0.15 mg/kg or 0.2 mg/kg administered once daily for a maximum of 5 days. Treatment beyond 5 days or for more than one course of therapy is not recommended. The first dose of rasburicase should be administered 4–24 h before starting chemotherapy. Rasburicase is given as an intravenous infusion over 30 min and should not be given as a bolus infusion. Currently, in the adult population, the dose often utilized in practice is 0.2 mg/kg.
Erythropoietin (EPO)
A growing body of evidence indicates that EPO has tissue-protective effects and prevents tissue damage during ischaemia and inflammation. Tissue protection after ischaemia and injury has been found in the brain, heart and kidney. It has been speculated that EPO has anti-apoptotic effects in cardiovascular cells. These novel effects of EPO seem to be independent of its erythropoietic activity (for reviews [116,117]). Recent in vitro and in vivo studies have demonstrated that EPO attenuates experimental renal ischaemic/toxic cell damage [118,119] and there is an extensive physiological basis for using EPO also in clinical AKI [120]. A recent major clinical trial in ICU patients with EPO-α, however, did not reduce the incidence of red-cell transfusion but showed a tendency to reduce mortality, in particular in trauma patients [121]. This trial did not evaluate AKI as outcome measure. A recent search of the clinical trial website (www.ClinTrials.gov) revealed that currently three trials are performed with EPO in the prevention of AKI post-cardiac surgery, CIN and post-kidney transplantation, respectively.
Conclusions
The two parts of this review evaluate the efficacy of a number of non-pharmacological and pharmacological preventative interventions in patients with risk for AKI.
The general prevention measures include the estimation of several risk factors for AKI, and the avoidance or minimization of nephrotoxic agents and, promising for the near future, diagnostic parameters to detect incipient renal damage before a measurable fall in GFR.
An important further preventative step, particularly in critically ill patients, is paying attention to the volume status and initiation of adequate fluid therapy. There is no convincing evidence that fluids other than isotonic saline are to be preferred. Maintenance of an adequate renal perfusion pressure with fluids and vasopressors remains the main nonpharmacological strategy to prevent AKI. Norepinephrine remains the vasopressor of choice in the management of hypotensive shock.
The many pharmacological interventions that have been used in the prevention of clinical AKI include vasodilators, diuretics, N-acetylcysteine, statins and vitamin C. Most of the clinical trials with these drugs have been tested in the prevention of radiocontrast-induced nephropathy and of post-cardiovascular surgery AKI. Except NAC, none of the other interventions were able to convincingly demonstrate that they are effective.
Although the clinical treatment of patients with ARF is still largely supportive, basic research continuously provides the clinician with many, albeit still unproved, approaches to future therapies. Additional experimental models that better reflect the multifactorial causes of clinical ARF are needed. In view of the different pathophysiologic processes involved during different stages in the initiation and maintenance of post-ischaemic ARF, it is clear that single-drug therapy will probably never be effective, and that multiple agents may be needed to improve outcomes. In addition, drugs should be administered early during the course of the disease.
The medical community should not be discouraged by the negative results of the many clinical trials, but should continuously think and rethink the basic and clinical strategies to improve the grim prognosis of this dreadful disease.
Conflict of interest statement. None declared.
References
- 1.Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008;36:S179–S186. doi: 10.1097/CCM.0b013e318169167f. [DOI] [PubMed] [Google Scholar]
- 2.Kellum JA, Pinsky MR. Use of vasopressor agents in critically ill patients. Curr Opin Crit Care. 2002;8:236–241. doi: 10.1097/00075198-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Langenberg C, Bellomo R, May C, et al. Renal blood flow in sepsis. Crit Care. 2005;9:R363–R374. doi: 10.1186/cc3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schetz M. Vasopressors and the kidney. Blood Purif. 2002;20:243–251. doi: 10.1159/000047016. [DOI] [PubMed] [Google Scholar]
- 5.Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med. 2006;34:589–597. doi: 10.1097/01.CCM.0000201896.45809.E3. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Papazian L, Perrin G, et al. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103:1826–1831. doi: 10.1378/chest.103.6.1826. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Viviand X, Leone M, et al. Effect of norepinephrine on the outcome of septic shock. Crit Care Med. 2000;28:2758–2765. doi: 10.1097/00003246-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth. 1998;12:567–586. doi: 10.1016/s1053-0770(98)90106-9. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Auriemma S, Fabbri A, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31:166–178. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Heywood JT. The confounding issue of comorbid renal insufficiency. Am J Med. 2006;119:S17–S25. doi: 10.1016/j.amjmed.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Kohli HS, Bhaskaran MC, Muthukumar T, et al. Treatment-related acute renal failure in the elderly: a hospital-based prospective study. Nephrol Dial Transplant. 2000;15:212–217. doi: 10.1093/ndt/15.2.212. [DOI] [PubMed] [Google Scholar]
- 12.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 13.Olsen PS, Schroeder T, Perko M, et al. Renal failure after operation for abdominal aortic aneurysm. Ann Vasc Surg. 1990;4:580–583. doi: 10.1016/S0890-5096(06)60843-1. [DOI] [PubMed] [Google Scholar]
- 14.Pascual J, Liano F. Causes and prognosis of acute renal failure in the very old. Madrid Acute Renal Failure Study Group. J Am Geriatr Soc. 1998;46:721–725. doi: 10.1111/j.1532-5415.1998.tb03807.x. [DOI] [PubMed] [Google Scholar]
- 15.Santacruz F, Barreto S, Mayor MM, et al. Mortality in elderly patients with acute renal failure. Ren Fail. 1996;18:601–605. doi: 10.3109/08860229609047683. [DOI] [PubMed] [Google Scholar]
- 16.Sharrock NE, Beksac B, Flynn E, et al. Hypotensive epidural anaesthesia in patients with preoperative renal dysfunction undergoing total hip replacement. Br J Anaesth. 2006;96:207–212. doi: 10.1093/bja/aei308. [DOI] [PubMed] [Google Scholar]
- 17.Sturm JT, Billiar TR, Luxenberg MG, et al. Risk factors for the development of renal failure following the surgical treatment of traumatic aortic rupture. Ann Thorac Surg. 1987;43:425–427. doi: 10.1016/s0003-4975(10)62821-4. [DOI] [PubMed] [Google Scholar]
- 18.Tallgren M, Niemi T, Poyhia R, et al. Acute renal injury and dysfunction following elective abdominal aortic surgery. Eur J Vasc Endovasc Surg. 2007;33:550–555. doi: 10.1016/j.ejvs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Wencker D. Acute cardio-renal syndrome: progression from congestive heart failure to congestive kidney failure. Curr Heart Fail Rep. 2007;4:134–138. doi: 10.1007/s11897-007-0031-4. [DOI] [PubMed] [Google Scholar]
- 20.Zanchetti A, Stella A. Cardiovascular disease and the kidney: an epidemiologic overview. J Cardiovasc Pharmacol. 1999;33(Suppl 1):S1–S6. doi: 10.1097/00005344-199900001-00001. [DOI] [PubMed] [Google Scholar]
- 21.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 22.Liu YL, Licari E, Uchino S, et al. Changes in blood pressure before the development of nosocomial acute kidney injury. Nephrol Dial Transplant. doi: 10.1093/ndt/gfn490. (in press) [DOI] [PubMed] [Google Scholar]
- 23.De Laet I, Malbrain ML, Jadoul JL, et al. Renal implications of increased intra-abdominal pressure: are the kidneys the canary for abdominal hypertension? Acta Clin Belg Suppl. 2007:119– 130. doi: 10.1179/acb.2007.62.s1.015. [DOI] [PubMed] [Google Scholar]
- 24.De Waele JJ, De Laet I. Intra-abdominal hypertension and the effect on renal function. Acta Clin Belg Suppl. 2007:371–374. doi: 10.1179/acb.2007.083. [DOI] [PubMed] [Google Scholar]
- 25.Malbrain ML, Chiumello D, Pelosi P, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–322. doi: 10.1097/01.ccm.0000153408.09806.1b. [DOI] [PubMed] [Google Scholar]
- 26.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 27.Parrillo JE. Septic shock—vasopressin, norepinephrine, and urgency. N Engl J Med. 2008;358:954–956. doi: 10.1056/NEJMe0800245. [DOI] [PubMed] [Google Scholar]
- 28.Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 29.Holmes CL, Walley KR. Arginine vasopressin in the treatment of vasodilatory septic shock. Best Pract Res Clin Anaesthesiol. 2008;22:275–286. doi: 10.1016/j.bpa.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RJ, Linas SL, Berns AS, et al. Nonoliguric acute renal failure. N Engl J Med. 1977;296:1134–1138. doi: 10.1056/NEJM197705192962002. [DOI] [PubMed] [Google Scholar]
- 31.Brivet FG, Kleinknecht DJ, Loirat P, et al. Acute renal failure in intensive care units—causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med. 1996;24:192–198. doi: 10.1097/00003246-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RL, Pascual MT, Soroko S, et al. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547–2553. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 33.Bagshaw SM, Delaney A, Jones D, et al. Diuretics in the management of acute kidney injury: a multinational survey. Contrib Nephrol. 2007;156:236–249. doi: 10.1159/000102089. [DOI] [PubMed] [Google Scholar]
- 34.Van Biesen W, Yegenaga I, Vanholder R, et al. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;18:54–60. [PubMed] [Google Scholar]
- 35.Sampath S, Moran JL, Graham PL, et al. The efficacy of loop diuretics in acute renal failure: Assessment using Bayesian evidence synthesis techniques. Crit Care Med. 2007 doi: 10.1097/01.ccm.0000284503.88148.6f. (Publish Ahead of Print) [DOI] [PubMed] [Google Scholar]
- 36.Bagshaw SM, Delaney A, Haase M, et al. Loop diuretics in the management of acute renal failure: a systematic review and meta-analysis. Crit Care Resusc. 2007;9:60–68. [PubMed] [Google Scholar]
- 37.Townsend DR, Bagshaw SM. New insights on intravenous fluids, diuretics and acute kidney injury. Nephron Clin Pract. 2008;109:c106–c116. doi: 10.1159/000142930. [DOI] [PubMed] [Google Scholar]
- 38.Lameire N, Vanholder R, Van Biesen W. Loop diuretics for patients with acute renal failure: helpful or harmful? JAMA. 2002;288:2599–2601. doi: 10.1001/jama.288.20.2599. [DOI] [PubMed] [Google Scholar]
- 39.Uchino S, Doig GS, Bellomo R, et al. Diuretics and mortality in acute renal failure. Crit Care Med. 2004;32:1669–1677. doi: 10.1097/01.ccm.0000132892.51063.2f. [DOI] [PubMed] [Google Scholar]
- 40.Better OS, Rubinstein I, Winaver JM, et al. Mannitol therapy revisited (1940–1997) Kidney Int. 1997;52:886–894. doi: 10.1038/ki.1997.409. [DOI] [PubMed] [Google Scholar]
- 41.Sever MS, Vanholder R, Lameire N. Management of crush-related injuries after disasters. N Engl J Med. 2006;354:1052–1063. doi: 10.1056/NEJMra054329. [DOI] [PubMed] [Google Scholar]
- 42.Vanholder R, Sever MS, Erek E, et al. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 43.Schetz M. Should we use diuretics in acute renal failure? Best Pract Res Clin Anaesthesiol. 2004;18:75–89. doi: 10.1016/j.bpa.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Yallop KG, Sheppard SV, Smith DC. The effect of mannitol on renal function following cardio-pulmonary bypass in patients with normal pre-operative creatinine. Anaesthesia. 2008;63:576–582. doi: 10.1111/j.1365-2044.2008.05540.x. [DOI] [PubMed] [Google Scholar]
- 45.Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 46.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259–265. doi: 10.1038/ki.1994.32. [DOI] [PubMed] [Google Scholar]
- 47.Reddy VG. Prevention of postoperative acute renal failure. J Postgrad Med. 2002;48:64–70. [PubMed] [Google Scholar]
- 48.Schnuelle P, Van Der Woude JF. Perioperative fluid management in renal transplantation: a narrative review of the literature. Transpl Int. 2006;19:947–959. doi: 10.1111/j.1432-2277.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 49.van Valenberg PL, Hoitsma AJ, Tiggeler RG, et al. Mannitol as an indispensable constituent of an intraoperative hydration protocol for the prevention of acute renal failure after renal cadaveric transplantation. Transplantation. 1987;44:784–788. doi: 10.1097/00007890-198712000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Weimar W, Geerlings W, Bijnen AB, et al. A controlled study on the effect of mannitol on immediate renal function after cadaver donor kidney transplantation. Transplantation. 1983;35:99–101. [PubMed] [Google Scholar]
- 51.Visweswaran P, Massin EK, DuBose TD., Jr Mannitol-induced acute renal failure. J Am Soc Nephrol. 1997;8:1028–1033. doi: 10.1681/ASN.V861028. [DOI] [PubMed] [Google Scholar]
- 52.Bellomo R, Chapman M, Finfer S, et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–2143. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- 53.Burton CJ, Tomson CR. Can the use of low-dose dopamine for treatment of acute renal failure be justified? Postgrad Med J. 1999;75:269–274. doi: 10.1136/pgmj.75.883.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denton MD, Chertow GM, Brady HR. ‘Renal-dose’ dopamine for the treatment of acute renal failure: scientific rationale, experimental studies and clinical trials. Kidney Int. 1996;50:4–14. doi: 10.1038/ki.1996.280. [DOI] [PubMed] [Google Scholar]
- 55.Friedrich JO, Adhikari N, Herridge MS, et al. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510–524. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- 56.Kellum JA, Decker M. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29:1526–1531. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Lauschke A, Teichgraber UK, Frei U, et al. ‘Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int. 2006;69:1669–1674. doi: 10.1038/sj.ki.5000310. [DOI] [PubMed] [Google Scholar]
- 58.Padmanabhan R. Renal dose dopamine—it's myth and the truth. J Assoc Physicians India. 2002;50:571–575. [PubMed] [Google Scholar]
- 59.Mathur VS, Swan SK, Lambrecht LJ, et al. The effects of fenoldopam, a selective dopamine receptor agonist, on systemic and renal hemodynamics in normotensive subjects. Crit Care Med. 1999;27:1832–1837. doi: 10.1097/00003246-199909000-00021. [DOI] [PubMed] [Google Scholar]
- 60.Landoni G, Biondi-Zoccai GG, Marino G, et al. Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2008;22:27–33. doi: 10.1053/j.jvca.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 61.Cogliati AA, Vellutini R, Nardini A, et al. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: a randomized clinical study. J Cardiothorac Vasc Anesth. 2007;21:847–850. doi: 10.1053/j.jvca.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 62.Roasio A, Lobreglio R, Santin A, et al. Fenoldopam reduces the incidence of renal replacement therapy after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:23–26. doi: 10.1053/j.jvca.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Brienza N, Malcangi V, Dalfino L, et al. A comparison between fenoldopam and low-dose dopamine in early renal dysfunction of critically ill patients. Crit Care Med. 2006;34:707–714. doi: 10.1097/01.CCM.0000201884.08872.A2. [DOI] [PubMed] [Google Scholar]
- 64.Morelli A, Ricci Z, Bellomo R, et al. Prophylactic fenoldopam for renal protection in sepsis: a randomized, double-blind, placebo-controlled pilot trial. Crit Care Med. 2005;33:2451–2456. doi: 10.1097/01.ccm.0000186413.04875.ef. [DOI] [PubMed] [Google Scholar]
- 65.Stone GW, McCullough PA, Tumlin JA, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA. 2003;290:2284–2291. doi: 10.1001/jama.290.17.2284. [DOI] [PubMed] [Google Scholar]
- 66.Candido R, Burrell LM, Jandeleit-Dahm KAM, Cooper ME. Vasoactive peptides and the kidney. In: Brenner BM, editor. Brenner & Rector's The Kidney. Philadelphia: Saunders and Elsevier; 2008. pp. 333–362. [Google Scholar]
- 67.Khairallah W, Merheb M, Chaiban J, et al. Hormones and the kidney. In: Schrier RW, editor. Diseases of the Kidney & Urinary Tract. Philadelphia: Wolters Kluwer/Lippincott Williams Wilkins; 2007. pp. 234–284. [Google Scholar]
- 68.Martinez-Rumayor A, Richards AM, Burnett JC, et al. Biology of the natriuretic peptides. Am J Cardiol. 2008;101:3–8. doi: 10.1016/j.amjcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 69.Rubattu S, Sciarretta S, Valenti V, et al. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens. 2008;21:733–741. doi: 10.1038/ajh.2008.174. [DOI] [PubMed] [Google Scholar]
- 70.Lewis J, Salem MM, Chertow GM, et al. Anaritide Acute Renal Failure Study Group Atrial natriuretic factor in oliguric acute renal failure. Am J Kidney Dis. 2000;36:767–774. doi: 10.1053/ajkd.2000.17659. [DOI] [PubMed] [Google Scholar]
- 71.Meyer M, Pfarr E, Schirmer G, et al. Therapeutic use of the natriuretic peptide ularitide in acute renal failure. Ren Fail. 1999;21:85–100. doi: 10.3109/08860229909066972. [DOI] [PubMed] [Google Scholar]
- 72.Sward K, Valsson F, Odencrants P, et al. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit Care Med. 2004;32:1310–1315. doi: 10.1097/01.ccm.0000128560.57111.cd. [DOI] [PubMed] [Google Scholar]
- 73.Mentzer RM, Jr, Oz MC, Sladen RN, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA Trial. J Am Coll Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 74.Witteles RM, Kao D, Christopherson D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2007;50:1835–1840. doi: 10.1016/j.jacc.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 75.Bagshaw SM, Ghali WA. Theophylline for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Arch Intern Med. 2005;165:1087–1093. doi: 10.1001/archinte.165.10.1087. [DOI] [PubMed] [Google Scholar]
- 76.Katholi RE, Taylor GJ, McCann WP, et al. Nephrotoxicity from contrast media: attenuation with theophylline. Radiology. 1995;195:17–22. doi: 10.1148/radiology.195.1.7892462. [DOI] [PubMed] [Google Scholar]
- 77.Wang A, Holcslaw T, Bashore TM, et al. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000;57:1675–1680. doi: 10.1046/j.1523-1755.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 78.Clarkson MR, Friedewald JJ, Eustace JA, et al. Acute kidney injury. In: Brenner BM, editor. Brenner & Rector's The Kidney. Philadelphia: Saunders and Elsevier; 2008. pp. 943–986. [Google Scholar]
- 79.Cummings BS, Schellmann RG. Pathophysiology of nephrotoxic cell injury. In: Schrier RW, editor. Diseases of the Kidney & Urinary Tract. Philadelphia: Wolters Kluwer/Lippincott Williams& Wilkins; 2007. pp. 962–985. [Google Scholar]
- 80.Edelstein CL, Schrier RW. Pathophysiology of ischemic acute renal injury. In: Schrier RW, editor. Diseases of the Kidney & Urinary Tract. Philadelphia: Wolters Kluwer/Lippincott Willams & Wilkins; 2007. pp. 930–961. [Google Scholar]
- 81.Lameire N, Vanholder R. Pathophysiologic features and prevention of human and experimental acute tubular necrosis. J Am Soc Nephrol. 2001;12(Suppl 17):S20–S32. [PubMed] [Google Scholar]
- 82.Lameire NH, Vanholder R. Pathophysiology of ischaemic acute renal failure. Best Pract Res Clin Anaesthesiol. 2004;18:21–36. doi: 10.1016/j.bpa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Lameire NH, Vanholder RC. Acute renal failure: Pathophysiology and Prevention. In: Davison AM, Cameron JS, Grünfeld J-P, et al., editors. Oxford Textbook of Clinical Nephrology. Oxford: Oxford University Press; 2005. pp. 1445–1464. [Google Scholar]
- 84.DiMari J, Megyesi J, Udvarhelyi N, et al. N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272:F292–F298. doi: 10.1152/ajprenal.1997.272.3.F292. [DOI] [PubMed] [Google Scholar]
- 85.Brady HR, Singer GG. Acute renal failure. Lancet. 1995;346:1533–1540. doi: 10.1016/s0140-6736(95)92057-9. [DOI] [PubMed] [Google Scholar]
- 86.McCullough PA. Radiocontrast-induced acute kidney injury. Nephron Physiol. 2008;109:61–72. doi: 10.1159/000142938. [DOI] [PubMed] [Google Scholar]
- 87.Newhouse JH, Kho D, Rao QA, et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191:376–382. doi: 10.2214/AJR.07.3280. [DOI] [PubMed] [Google Scholar]
- 88.Tepel M, Van Der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 89.Fishbane S. N-Acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008;3:281–287. doi: 10.2215/CJN.02590607. [DOI] [PubMed] [Google Scholar]
- 90.Marenzi G, Assanelli E, Marana I, et al. N-Acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 91.Alonso A, Lau J, Jaber BL, et al. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1–9. doi: 10.1053/j.ajkd.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Birck R, Krzossok S, Markowetz F, et al. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003;362:598–603. doi: 10.1016/S0140-6736(03)14189-X. [DOI] [PubMed] [Google Scholar]
- 93.Isenbarger DW, Kent SM, O’Malley PG. Meta-analysis of randomized clinical trials on the usefulness of acetylcysteine for prevention of contrast nephropathy. Am J Cardiol. 2003;92:1454–1458. doi: 10.1016/j.amjcard.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 94.Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295:2765–2779. doi: 10.1001/jama.295.23.2765. [DOI] [PubMed] [Google Scholar]
- 95.Kelly AM, Dwamena B, Cronin P, et al. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284–294. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- 96.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 97.Hoffmann U, Fischereder M, Kruger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407–410. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- 98.Genet S, Kale RK, Baquer NZ. Effects of free radicals on cytosolic creatine kinase activities and protection by antioxidant enzymes and sulfhydryl compounds. Mol Cell Biochem. 2000;210:23–28. doi: 10.1023/a:1007071617480. [DOI] [PubMed] [Google Scholar]
- 99.Rehman T, Fought J, Solomon R. N-Acetylcysteine effect on serum creatinine and cystatin C levels in CKE patients. c JASN. 2008;3:1610–1614. doi: 10.2215/CJN.01560408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lameire N. Contrast-induced nephropathy in the critically-ill patient: focus on emergency screening and prevention. Acta Clin Belg Suppl. 2007;62:346–352. doi: 10.1179/acb.2007.078. [DOI] [PubMed] [Google Scholar]
- 101.Sinert R, Doty CI. Evidence-based emergency medicine review. Prevention of contrast-induced nephropathy in the emergency department. Ann Emerg Med. 2007;50:335–345. doi: 10.1016/j.annemergmed.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 102.Baker CS, Wragg A, Kumar S, et al. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol. 2003;41:2114–2118. doi: 10.1016/s0735-1097(03)00487-x. [DOI] [PubMed] [Google Scholar]
- 103.Adabag AS, Ishani A, Koneswaran S, et al. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J. 2008;155:1143–1149. doi: 10.1016/j.ahj.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 104.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 105.Khanal S, Attallah N, Smith DE, et al. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med. 2005;118:843–849. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 106.Patti G, Nusca A, Chello M, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008;101:279–285. doi: 10.1016/j.amjcard.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 107.Kor DJ, Brown MJ, Iscimen R, et al. Perioperative statin therapy and renal outcomes after major vascular surgery: a propensity-based analysis. J Cardiothorac Vasc Anesth. 2008;22:210–216. doi: 10.1053/j.jvca.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 108.Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110:2837–2842. doi: 10.1161/01.CIR.0000146396.19081.73. [DOI] [PubMed] [Google Scholar]
- 109.Briguori C, Airoldi F, D’Andrea D, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 110.Marenzi G, Marana I, Lauri G, et al. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003;349:1333–1340. doi: 10.1056/NEJMoa023204. [DOI] [PubMed] [Google Scholar]
- 111.Marenzi G, Lauri G, Campodonico J, et al. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med. 2006;119:155–162. doi: 10.1016/j.amjmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Lee PT, Chou KJ, Liu CP, et al. Renal protection for coronary angiography in advanced renal failure patients by prophylactic hemodialysis. A randomized controlled trial. J Am Coll Cardiol. 2007;50:1015–1020. doi: 10.1016/j.jacc.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 113.Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48:361–371. doi: 10.1053/j.ajkd.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 114.Cruz DN, Perazella MA, Ronco C. The role of extracorporeal blood purification therapies in the prevention of radiocontrast-induced nephropathy. Int J Artif Organs. 2008;31:515–524. doi: 10.1177/039139880803100607. [DOI] [PubMed] [Google Scholar]
- 115.Lameire NH, Flombaum CD, Moreau D, et al. Acute renal failure in cancer patients. Ann Med. 2005;37:13–25. doi: 10.1080/07853890510007205. [DOI] [PubMed] [Google Scholar]
- 116.Sturiale A, Campo S, Crasci E, et al. Experimental models of acute renal failure and erythropoietin: what evidence of a direct effect? Ren Fail. 2007;29:379–386. doi: 10.1080/08860220701193290. [DOI] [PubMed] [Google Scholar]
- 117.Veys N, Van BW, Lameire N. Internal medicine, renal anaemia, and erythropoiesis-stimulating agents (ESAS) Acta Clin Belg. 2007;62:396–407. doi: 10.1179/acb.2007.059. [DOI] [PubMed] [Google Scholar]
- 118.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 119.Yokomaku Y, Sugimoto T, Kume S, et al. Asialoerythropoietin prevents contrast-induced nephropathy. J Am Soc Nephrol. 2008;19:321–328. doi: 10.1681/ASN.2007040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bernhardt WM, Eckardt KU. Physiological basis for the use of erythropoietin in the critically ill patient at risk for acute kidney injury. Curr Opin Crit Care. doi: 10.1097/MCC.0b013e328317ee82. (in press) [DOI] [PubMed] [Google Scholar]
- 121.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epo- etin alfa in critically ill patients. N Engl J Med. 2007;357:965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]