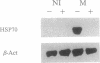
Abstract
We examined the effect of Listeria monocytogenes infection of J774 macrophage-like mouse cells on induction of several stress genes, including genes for heat shock proteins (HSPs) and a protein-tyrosine phosphatase (PTP), to understand the host response in various steps of the bacterial invasion process. Exposure to wild-type L. monocytogenes strain EGD elicited an early induction of HSP70 mRNA with a corresponding early appearance of HSP70 protein. Cytochalasin D pretreatment prevented the induction of HSP70 mRNA in L. monocytogenes-infected macrophages. After a 2-hr infection with L. monocytogenes, PTP and to a lesser extent HSP90 mRNA levels were elevated. A listeriolysin-negative mutant of L. monocytogenes strain EGD and a noninvasive species of Listeria, Listeria innocua, did not induce PTP or HSP90 mRNA in infected macrophages. Mutations in other virulence genes did not affect transcription of PTP or HSP90. Expression of HSP60 mRNA remained constant over the time course studied in wild-type or mutant strains. These results suggest that phagocytosis of L. monocytogenes triggers transcription of HSP70 mRNA in macrophages; however, escape from the phagosome appears to be necessary for induction of PTP and HSP90 mRNA. Since both PTP and HSP90 may have links with signal transduction pathways in eukaryotic cells, the induction of these mRNAs suggests a role for L. monocytogenes in influencing the signal transduction routes of the host cell.
Full text
PDF

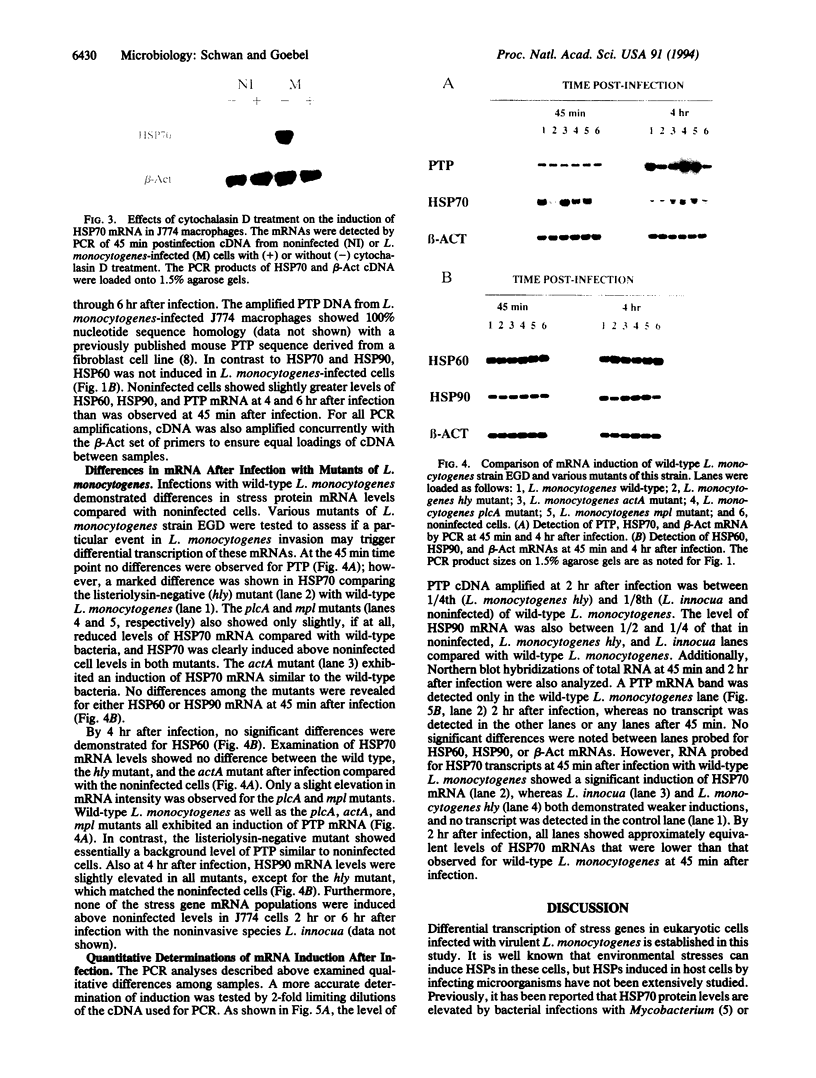
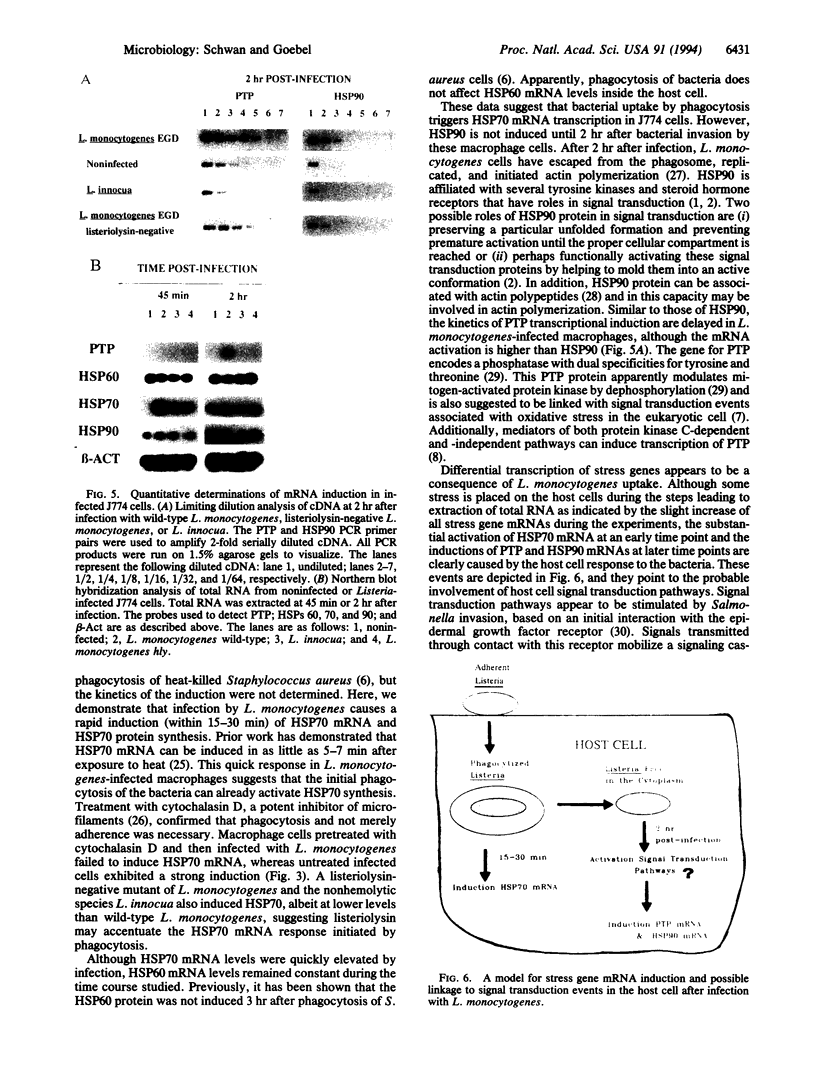

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D. R., Smythe C., Keyse S. M. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993 Jul;8(7):2015–2020. [PubMed] [Google Scholar]
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- Charles C. H., Abler A. S., Lau L. F. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992 Jan;7(1):187–190. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clerget M., Polla B. S. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1081–1085. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gambill B. D., Nelson R. J. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993 Jun;57(2):402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Broyles S. S., Dixon J. E. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature. 1991 Mar 28;350(6316):359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- Kantengwa S., Polla B. S. Phagocytosis of Staphylococcus aureus induces a selective stress response in human monocytes-macrophages (M phi): modulation by M phi differentiation and by iron. Infect Immun. 1993 Apr;61(4):1281–1287. doi: 10.1128/iai.61.4.1281-1287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Emslie E. A. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992 Oct 15;359(6396):644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Nishida E., Kadowaki T., Matsuzaki F., Iida K., Harada F., Kasuga M., Sakai H., Yahara I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8054–8058. doi: 10.1073/pnas.83.21.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Induction of cytokines in phagocytic mammalian cells infected with virulent and avirulent Listeria strains. Infect Immun. 1994 Feb;62(2):348–356. doi: 10.1128/iai.62.2.348-356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Kathariou S., Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 Jan;56(1):79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Moran L. A. Molecular cloning and analysis of DNA complementary to three mouse Mr = 68,000 heat shock protein mRNAs. J Biol Chem. 1986 Feb 15;261(5):2102–2112. [PubMed] [Google Scholar]
- Mistry Y., Young D. B., Mukherjee R. hsp70 synthesis in Schwann cells in response to heat shock and infection with Mycobacterium leprae. Infect Immun. 1992 Aug;60(8):3105–3110. doi: 10.1128/iai.60.8.3105-3110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. K., Kozak C., Robinson E. A., Ullrich S. J., Appella E. Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem. 1989 Apr 5;264(10):5343–5351. [PubMed] [Google Scholar]
- Pace J., Hayman M. J., Galán J. E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993 Feb 26;72(4):505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Sauer R. T. Induction of a heat shock-like response by unfolded protein in Escherichia coli: dependence on protein level not protein degradation. Genes Dev. 1989 Aug;3(8):1226–1232. doi: 10.1101/gad.3.8.1226. [DOI] [PubMed] [Google Scholar]
- Picketts D. J., Mayanil C. S., Gupta R. S. Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins. J Biol Chem. 1989 Jul 15;264(20):12001–12008. [PubMed] [Google Scholar]
- Platzer C., Richter G., Uberla K., Müller W., Blöcker H., Diamantstein T., Blankenstein T. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur J Immunol. 1992 May;22(5):1179–1184. doi: 10.1002/eji.1830220511. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Chakraborty T., Goebel W., Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992 Apr;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Schwan W. R., Seifert H. S., Duncan J. L. Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J Bacteriol. 1992 Apr;174(7):2367–2375. doi: 10.1128/jb.174.7.2367-2375.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Tilney M. S. The wily ways of a parasite: induction of actin assembly by Listeria. Trends Microbiol. 1993 Apr;1(1):25–31. doi: 10.1016/0966-842x(93)90021-i. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]