Abstract
We report the staggered clinical course of a young Caucasian female who suffered rare deleterious effects of Nurofen Plus misuse with a near fatal outcome. Several life-threatening events intervened before the underlying problem of serious dependency was identified. Effects on renal tubular acidification and bone marrow function as well as the commoner complications of acute kidney injury and peptic ulceration are described. In addition, this is the first case report in which the syndrome of reversible posterior leucoencephalopathy is linked to analgesic misuse, occurring after recovery of renal function. Recommendations for restricting availability of codeine-based analgesics are made.
Keywords: acute kidney injury, non-steroidal anti inflammatory drugs, renal tubular acidosis, reversible posterior leucoencephalopathy
Introduction
The addictive potential of Nurofen Plus due to its codeine component has been a topic of considerable debate in recent years with regard to justifying its availability as a non-prescription medication. We report an example of life-threatening analgesic toxicity with rare complications as a consequence of Nurofen Plus dependency.
Case Report
A 29-year-old Caucasian female was referred to the hospital with acute kidney injury (AKI). She presented with cough, anorexia and vomiting, and was afebrile. Serum creatinine was 902 µmol/L and urea 20 mmol/L. Relevant past history included a diagnosis of renal tubular acidosis (RTA), peptic ulcer disease (PUD) and depression. The AKI was presumed to be multifactorial, with dehydration, an underlying respiratory infection and recent use of non-steroidal anti inflammatory drugs (NSAIDs) for a toothache contributing. Failing to recover with fluid repletion, she required short-term haemodialysis with ongoing potassium, calcium and magnesium replacement.
Despite prompt recovery of renal function, our patient’s hospital course became quite complicated. Her haemoglobin fell to 7 g/dL shortly after admission, and after transfusion of 2 units of red blood cells, dropped further to 6.1 g/dL during the following week. Blood film revealed an inadequate reticulocyte response but no evidence of haemolysis. Anaemia was attributed to an extensive groin haematoma that developed following removal of a temporary femoral vein dialysis catheter and blood loss from a prepyloric ulcer noted on upper gastrointestinal endoscopy.
The patient subsequently developed a severe headache with visual hallucinations, confusion and transient visual loss followed by two tonic clonic seizures. Computerized tomography (CT) of the brain was unremarkable; however, brain magnetic resonance imaging (MRI) showed diffuse white matter changes predominantly in the posterior lobes. Phenytoin was commenced, and she was intubated for a short period in the intensive care unit (ICU) following a third seizure. Significant hypertension ensued. A diagnosis of reversible posterior leucoencephalopathy syndrome (RPLS) was made based on the typical clinical and radiological findings.
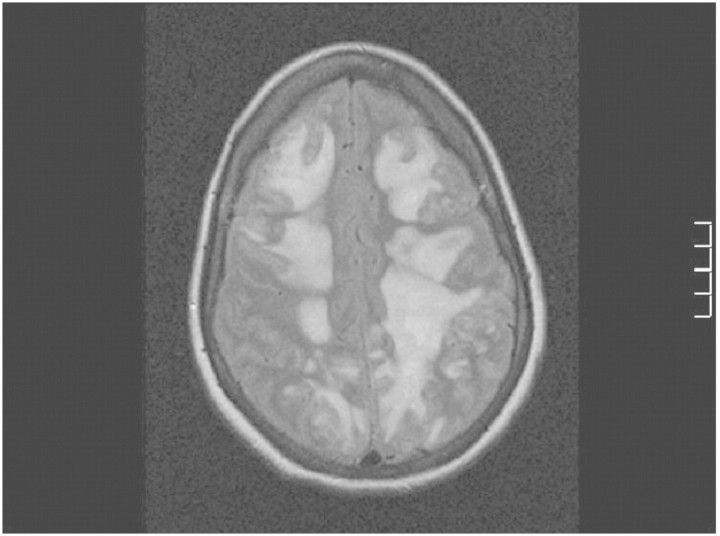
A history of excessive use of Nurofen Plus for chronic headaches was disclosed, and the patient admitted to self-medicating with it while in hospital. She reported taking up to 12 tablets daily for several years and had taken 24 tablets (4800 mg of ibuprofen, 307 mg of codeine) over a 36-h period in hospital prior to the onset of neurological symptoms. It was suspected that her consumption could have been greater than that stated. Five days later, she deteriorated with further seizures. A repeat brain CT showed diffuse cerebral oedema with imminent coning. Progressive signal abnormalities were seen on brain MRI (Figure 1). She was readmitted, unconscious, to the ICU, where she was intubated and hyperventilated for 8 days. In addition to dexamethasone, anticonvulsants and antihypertensives, broad spectrum antimicrobials were administered, although there was no evidence of an infective process.
Fig. 1.
Fluid-attenuated inversion recovery (FLAIR) MRI image on 22 October 2009 showing bilateral hyperintense signal abnormalities.
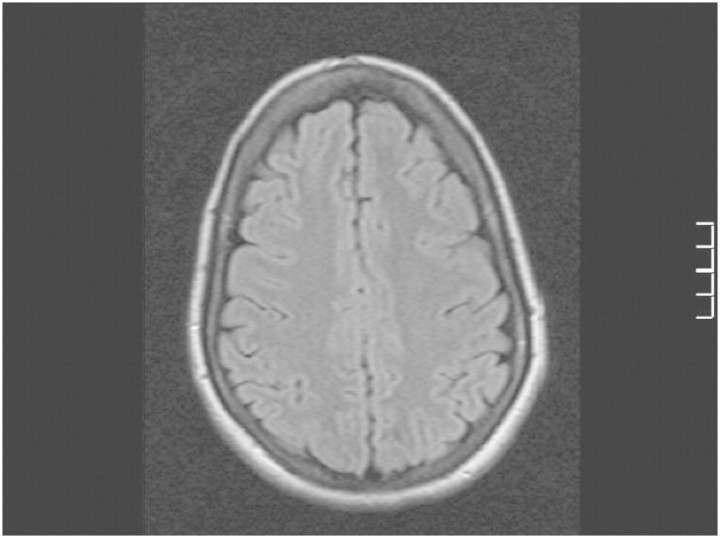
Marked clinical and radiological improvements were seen after 1week of supportive care, however, with residual cognitive deficits. There were major psychosocial stressors in this patient's social history. Neuropsychiatric assessment did not indicate any tendency to self-harm or suicidal ideation. Significant executive dysfunction with disinhibition, disengagement and impulsiveness was detected. It was estimated that these may take months to resolve, and some may persist indefinitely. Brain MRI, 3 months later, was normal (Figure 2).
Fig. 2.
FLAIR MRI image on 4 February 2010 showing complete resolution of high signal abnormalities.
Discussion
This case demonstrates how a vulnerable patient suffered a full-blown picture of analgesic misuse, from analgesic rebound headaches and dependency to cerebral oedema with imminent death and residual cognitive deficits. Nurofen Plus tablets contain the two active ingredients: ibuprofen and codeine phosphate. Codeine is the least habit forming of the opioids; nevertheless, prolonged use may induce physiological and psychological dependency. As addicts develop progressive tolerance to higher doses of codeine, they are simultaneously exposed to toxic doses of ibuprofen. This is also true for products that combine codeine with paracetamol for example, leading to paracetamol-induced liver toxicity in overdose.
In this case, Nurofen Plus dependency resulted in multiple organ toxicity from ibuprofen. The patient was severely anaemic while iron replete. Apart from the significant gastrointestinal toxicity, bone marrow suppression and platelet dysfunction attributable to ibuprofen may have contributed to her morbidity. While renal impairment is a well-recognized feature of NSAID overuse, RTA in association with these drugs has been described in only three previous case reports [1–3]. Our patient had a protracted course of illness with two previous hospitalizations due to life-threatening hypokalaemia in the preceding 2 years. She was diagnosed with RTA at the age of 28, when she presented with a serum potassium of 1.7 mmol/L and a normal anion gap metabolic acidosis. Transtubular potassium gradient was 12. Serum potassium and bicarbonate levels 5 years prior to that presentation were found to be normal. Features favouring a diagnosis of type 1 RTA were a persistently high urinary pH (≥6) and a positive urinary anion gap (+9 mmol/L). On the other hand, apart from a fall in serum bicarbonate to 7 mmol/L on one occasion, bicarbonate levels in the absence of renal failure were above 12 mmol/L as would be typical of type 2 RTA. Two previous reports associated ibuprofen overuse with type 2 RTA, and a separate paper described what was perceived to be a pre-existing type 1 RTA aggravated by the development of a proximal tubulopathy secondary to misuse of an ibuprofen–codeine combination product [4]. Although initially classified as an idiopathic RTA, in retrospect, it is likely that our patient’s RTA was a consequence of longstanding undisclosed NSAID overuse. Long-term follow-up is required to ascertain irreversibility of the tubular acidification defect.
The aetiology and pathogenesis of RPLS are still not clearly defined, and to our knowledge, neither NSAIDs nor codeine has previously been implicated in its aetiology in adults, although one paper described its association with NSAID use in a child [5]. The syndrome was first defined as a clinical entity in 1996 in a case series of 15 patients who were either immunosuppressed or had hypertensive encephalopathy associated with renal disease or eclampsia [6]. Headache, confusion, seizures and visual disturbance are the cardinal clinical features constituting the syndrome as originally described, in association with predominantly posterior white matter changes on neuroimaging. Since then, it has been identified in a variety of clinical settings, many of which are pertinent to nephrology practice.
Our patient developed RPLS in association with Nurofen Plus toxicity. It is noteworthy that significant hypertension occurred following the onset of the syndrome with its characteristic radiological findings and hence did not appear to be the triggering factor in this case. Average systolic blood pressure in the 24-h period preceding the first seizure was 137 mmHg and diastolic blood pressure 84 mmHg with no dramatic fluctuations. Likewise, although RPLS is associated with renal impairment and haemodialysis [7], a week had elapsed between the last haemodialysis and the onset of symptoms when renal function was recovering to baseline (creatinine 130 µmol/L and urea 3.6 mmol/L). At the time of recurrence of seizure activity with further clinical and radiological deterioration, creatinine was 84 µmol/L, and urea was 1.8 mmol/L. Lower magnesium levels of 0.5 mmol/L coincided with seizure onset and recurrence, possibly lowering the seizure threshold. RPLS is reported to occur in the setting of massive blood transfusion [8–10]. In this case, the patient received only four units of red blood cells; two were transfused the day preceding the first seizure.
While it is conceivable that the aforementioned factors could have contributed to her leucoencephalopathy, the patient developed the syndrome at a time when she reported first taking Nurofen Plus in hospital. Ibuprofen is rapidly metabolized with urinary excretion of the inactive metabolites usually complete within 24 h; peak serum levels usually occur within 2 hours. Ibuprofen was detected in the patient’s serum 30 h following recurrence of seizure activity and deterioration of neurological status, confirming recent ingestion (ibuprofen level 36 mg/L). We therefore propose a direct association between the syndrome and ibuprofen toxicity.
It is postulated that toxic levels of ibuprofen led to vasogenic cerebral oedema through Leukotriene-mediated endothelial leakage or via a direct adverse effect on endothelial function. By inhibiting cyclooxygenase, NSAIDs, particularly in excessive doses, result in increased availability of arachidonic acid for the Leukotriene-producing 5-lipoxygenase pathway.
With this report, we highlight the potential magnitude of self-harm from Nurofen Plus misuse and recommend a system for monitoring the supply of codeine-containing medications (CCM) to the public in Ireland, where they are currently sold over the counter (OTC) in pharmacies. We conclude that compound analgesics containing a nephrotoxic substance plus another drug creating an addiction should not be available for public use without restrictions as this causes an underestimation of their adverse effects. A web-based CCM database could be devised, whereby pharmacies can monitor the purchase of these medications OTC. The system would obviate unnecessary doctor visits and the seeking of prescriptions as it has always been argued that millions use these medications legitimately and sensibly. For first time use, however, a particular patient should be registered onto the system by his/her doctor.
This recommendation comes at a time when the Pharmaceutical Society of Ireland has issued new draft guidelines due to come into effect this year, whereby pharmacies should store CCM in the dispensaries and not display them to the public.
Conflict of interest statement. None declared.
References
- 1.Gaul C, Heckmann JG, Druschky A, et al. Renal tubular acidosis with severe hypokalemic tetraparesis after ibuprofen intake. Dtsch Med Wochenschr. 1999;124:483–486. doi: 10.1055/s-2007-1024347. [DOI] [PubMed] [Google Scholar]
- 2.Lambert AP, Close C. Life-threatening hypokalaemia from abuse of Nurofen Plus. J R Soc Med. 2005;98:21. doi: 10.1258/jrsm.98.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chetty R, Baoku Y, Mildner R, et al. Severe hypokalemia and weakness due to Nurofen misuse. Ann Clin Biochem. 2003;40:422–423. doi: 10.1258/000456303766477101. [DOI] [PubMed] [Google Scholar]
- 4.Ter A, Salha R, Vadamalai V, et al. Ibuprofen codeine combination precipitating severe hypokalaemia in a patient with pre-existing type 1 renal tubular acidosis. NDT Plus. 2008;4:270–271. doi: 10.1093/ndtplus/sfn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokobori S, Yokota H, Yamamoto Y. Pediatric posterior reversible leukoencephalopathy syndrome and NSAID-induced acute tubular interstitial nephritis. Pediatr Neurol. 2006;34:245–247. doi: 10.1016/j.pediatrneurol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 7.Kevin N, Gregory F, Steven R, et al. Dialysis disequilibrium: another reversible posterior leukoencephalopathy syndrome? Clin Neurol Neurosurg. 2003;105:249–252. doi: 10.1016/s0303-8467(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Niwa H, Iida T, et al. Post-transfusion reversible posterior leukoencephalopathy syndrome with cerebral vasoconstriction. Neurology. 1997;49:1174. doi: 10.1212/wnl.49.4.1174. [DOI] [PubMed] [Google Scholar]
- 9.Heo K, Park SA, Lee JY, et al. Post-transfusion posterior leukoencephalopathy with cytotoxic and vasogenic edema precipitated by vasospasm. Cerebrovasc Dis. 2003;15:230. doi: 10.1159/000068825. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Tsai P, Yeh J, et al. Reversible posterior leukoencephalopathy syndrome caused by blood transfusion: a case report. Acta neurologica Taiwanica. 2008;17:258–262. [PubMed] [Google Scholar]