Abstract
Cytomegalovirus (CMV) infection involving the skin is rare. We present a case of a renal transplant recipient who developed fever and axillary, scrotal and penile skin ulcers after renal transplantation. The skin ulcers did not heal with antibiotics. The skin biopsy revealed CMV inclusion bodies, CMV antigen on immunohistochemistry and high CMV DNA copies. The patient was diagnosed with CMV cutaneous ulcers. The skin ulcers healed after treatment with intravenous ganciclovir and oral valganciclovir. The diagnosis of CMV disease should be considered in the febrile immunosuppressed patient with skin ulcers. The biopsy of the cutaneous lesions may provide the diagnostic clue in such patients.
Keywords: cytomegalovirus infection, renal allograft recipient, skin ulcers
Backgrounds
Cytomegalovirus (CMV) is a common viral infection in renal transplant recipients, affecting more than 50% of recipients of major organ transplants [1]. The most common clinical presentation is a viral syndrome characterized by fever, leucopenia, thrombocytopenia, atypical lymphocytosis and other well-documented manifestations including pneumonia, liver dysfunction and gastrointestinal disease [2]. Skin infection with CMV is rare. Here, we report an unusual case of a renal alograft recipient who developed ulcers over the right axilla, scrotum and penile skin not responding to antibiotics, and the skin biopsies from the axillary and scrotal ulcers revealed several enlarged endothelial cells of the dermal blood vessels containing intranuclear and intracytoplasmic inclusions positive for CMV antigen on immunohistochemistry. The patient responded to anti-CMV therapy.
Case report
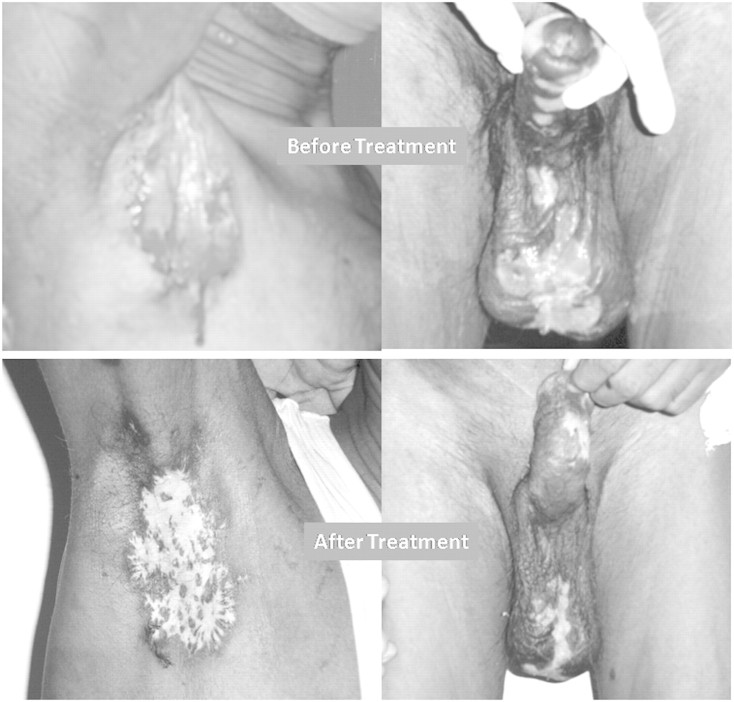
A 52-year-old, live related renal allograft recipient, after a 2.5-month post-transplant period, presented with a history of vesicular eruptions over the right axilla, scrotum and penile skin which later developed into ulcers (Figure 1). Both the donor and the recipient were CMV immunoglobulin G (IgG) antibody positive and CMV IgM antibody negative before transplantation. He was on triple immunosuppression mycophenolate mofetil, cyclosporine and prednisolone. He developed new-onset diabetes in the immediate post-transplant period for which he was on insulin therapy. He had C4d-negative acute graft rejection after 20 days of transplant, which was treated with three doses of methyl prednisolone and graft function become normal. On presentation, he had pyrexia of 38.5°C, pulse rate 90/min and BP 130/84 mmHg, and other physical examinations were within normal limits. Laboratory findings revealed a haemoglobin of 10.5 g/dL, total leucocyte count of 17 000/µL with 83% neutrophils, platelet count of 310 × 103/mm3, fasting blood glucose of 69 mg/dL, serum creatinine of 1.71 mg/dL and normal liver function tests. His urine examination showed mild proteinuria and two to four leucocytes per high-power field. Blood and urine cultures were sterile. A chest radiograph was normal. A swab culture from the axillary and scrotal ulcers revealed Pseudomonas infection. The patient was treated with intravenous (IV) cefoperazone–sulbactam combination antibiotic for 7 days. The ulcers were not healing and fever was persisting. Subsequently, the patient was treated with another set of antibiotics, cefuroxime and piperacillin–tazobactam combination for further 7 days. The skin ulcers and pyrexia did not respond to this therapy either and antibiotic treatment was discontinued. Then skin biopsy was done and samples were sent for microbiology and histology. The total leucocyte count at this time was 12 500/µL with 72% neutrophils, and serum creatinine was 1.86 mg/dL. Cultures from the axillary and scrotal ulcers were sterile. The skin biopsies from the axillary and scrotal ulcers revealed intranuclear and intracytoplasmic inclusions in several enlarged endothelial cells of the dermal blood vessels (Figure 2). These inclusions were positivity for CMV antigen (DAKO) on immunohistochemistry (Figure 3). Serology tests yielded CMV IgG positivity, and CMV viral load as measured by quantitative real-time polymerase chain reaction was 66 900 copies/mL. The patient was treated with IV ganciclovir 2.5 mg/kg at 12-h intervals for 14 days and then the patient was discharged with advice to continue oral valganciclovir 450 mg daily for 2.5 months as maintenance therapy. He became afebrile with healing and regression of the skin ulcers after 2 weeks of ganciclovir therapy (Figure 1). At the time of discharge, his serum creatinine was 1.61 mg/dL. The intensity of immunosuppression was reduced during the period of CMV treatment by reducing the dose of cyclosporine. On a follow-up visit 3 months later, the patient was completely asymptomatic with a total leucocyte count of 4800/µL and serum creatinine of 1.38 mg/dL. The presence of CMV inclusion bodies, presence of CMV antigen on immunohistochemistry, high CMV DNA copies per millilitre and response to anti-CMV therapy indicated CMV as primary causative organism of ulcer in this patient.
Fig. 1.
Photograph of axillary and scrotal ulcer before and after treatment.
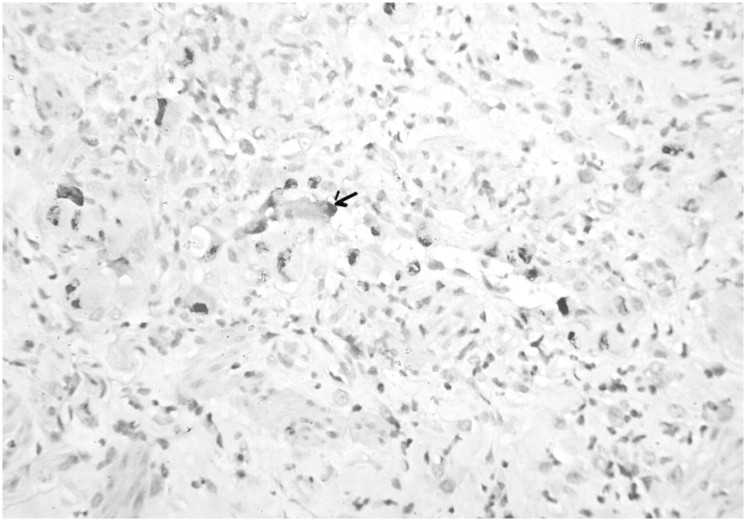
Fig. 2.
Microphotograph of biopsy from axillary ulcer showing endothelial cells with intranuclear and intracytoplasmic inclusions (arrow) (H&E, ×400).
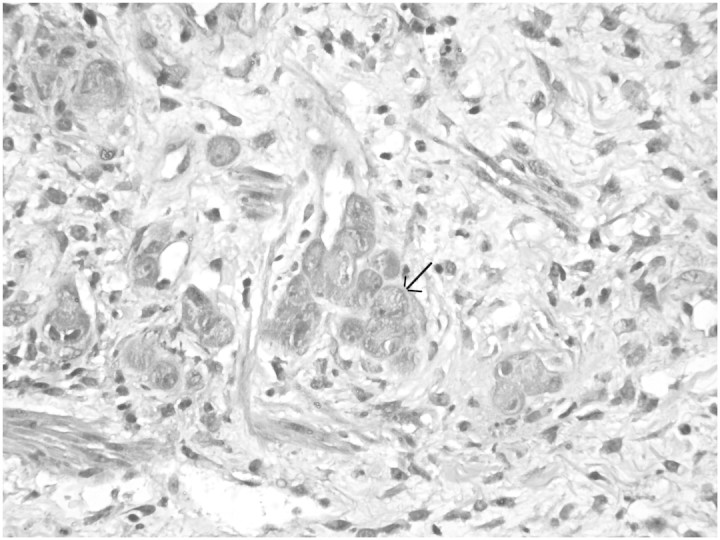
Fig. 3.
Immunohistochemistry for CMV antigen showing nuclear positivity in the endothelial cells of dermal blood vessels (arrow) (×200).
Discussion
In this case study, we report a very rare manifestation of CMV disease, a cutaneous ulcers, in a live related renal transplant recipient. The usual clinical features of active disseminated CMV disease include fever, leucopenia, thrombocytopenia, petechiae, pneumonitis, hepatitis, chorioretinitis, adrenalitis and encephalitis [3]. Skin involvement is a rare manifestation of CMV disease in kidney transplantation [4]. However, ulcers, mostly around genitalia, perineum, buttocks and thighs, have been described in immunocompromised patients particularly in acquired immunodeficiency syndrome (AIDS) patients [5]. However, to our knowledge, axillary ulcers have not been described in CMV-associated cutaneous lesions in renal allograft recipients. Recently, the true pathogenetic role of CMV in cutaneous lesions has been the focus of interest. It has been thought that CMV may be simply an innocent bystander during herpes simplex virus infection among non-AIDS patients [6]. But the demonstration of CMV antigen on immunohistochemistry, CMV inclusion bodies, high titre of CMV DNA and overall response to anti-CMV therapy with ganciclovir and valganciclovir suggest the true pathogenetic role of CMV in cutaneous ulcers in renal transplant recipients. One should always consider a differential diagnosis of CMV skin ulcers in a non-healing ulcers in renal transplant recipients. Choi et al. [6] have also shown a pathogenetic role of CMV in cutaneous lesions, it is not simply a bystander in skin ulcers. Choi et al. [6] have reported nine cases (five haematological and four organ recipients) of CMV skin lesion in nine non-AIDS immunocompromised patients; six of them had multiple anogenital ulceration, two had a solitary nodule and one had maculopapular rash. CMV usually causes intracellular lesion by nature and ulceration of a lesion is not common. Lee et al. [7] have also reported three cases of CMV skin ulcers in transplant recipients; the first case was associated with extravasated inotrope-induced skin ulcers, second with Candida infection and third with pneumonia. Of the three cases, two died and only one survived. Lee et al. [7] have shown that CMV infection, when detected in the skin, appears to be associated with grave prognosis and reported mortality is 85%, but the fatal cases had either concurrent disseminated CMV infection or mixed cutaneous or systemic infections. When the infection is localized in the skin wounds, the prognosis seems fairly good. Our patient responded well to treatment and survived.
The high leucocyte count and growth of Pseudomonas from the wound at presentation was misleading concerning the diagnosis of CMV and the patient was treated with antibacterial agents. It is known that CMV is an immunomodulatory virus and predisposes the patients to other associated opportunistic bacterial, viral and fungal infection. Our patient also had associated Pseudomonas infection which may be the reason of the high leucocyte count in this patient contradictory to the usual presentation of leucopenia in CMV infection. Recently, Roco et al. [8] reported a case of ureterostomy cytomegalovirus infection presenting as stoma ulceration. The summary of the review of literature of CMV skin ulcers in renal transplant recipients is shown in Table 1.
Table 1.
Summary of review of literature of CMV cutaneous ulcers in organ recipients
| Studies | No. of cases | Manifestations | Outcome |
|---|---|---|---|
| Lee et al. [6] | 3 transplant recipients | Case 1, wound due to extravasations of inotropes and associated CMV | Survived |
| Case 2, mixed Candida and CMV | Died due to disseminated herpes simplex virus | ||
| Case 3, CMV pneumonia and purpuric rash | Died of Pseudomonas sepsis | ||
| Choi et al. [7] | Non-AIDS immunocompromised patients | Multiple anogenital ulcerations—6/9 | 6 survived and 3 died |
| Total 9 cases—5 haematological causes, 4 organ recipients (2 liver and 2 kidney recipients) | Solitary nodule—2/9 | Both kidney recipients survived | |
| Maculopapular rash—1/9 | |||
| Both kidney recipients have perineal and penile shaft lesion | |||
| Only one has coexisting HSV infection | |||
| Rico et al. [8] | Transplant recipient—1 | Ureterostomy cytomegalovirus infection presenting as stoma ulceration | Survived |
CMV infection is caused by a virus of the family Herpesviridae and is usually latent and asymptomatic in those with a normal immune system. Infection by CMV may be frequent and severe in children or adults with congenital or acquired defects of cellular immunity, with underlying malignancy, patients on immunosuppression and AIDS patients. There are three major patterns of CMV infection in transplant patients: (i) Primary infection occurs in seronegative recipient from seropositive donor. (ii) Reactivation of latent virus occurs in seropositive donor after transplant. (iii) Super infection occurs when allograft from a seropositive donor is transplanted into a seropositive recipient. Both our recipient and donor were seropositive and CMV disease appears to be due to either reactivation or super infection of CMV in this patient. Our patient had not been given ganciclovir prophylaxis as per our institute protocol. We used to give ganciclovir prophylaxis if a seronegative recipient receives a kidney from a seropositive donor and with the use of anti-thymocyte globulin for either induction purpose or for the treatment of rejection.
Therapeutic treatment of established CMV disease is primarily with the antiviral agent Ganciclovir IV induces remission of CMV disease in renal transplant recipients in all but rare cases that are ganciclovir resistant. Ganciclovir-resistant strains of CMV are generally sensitive to foscarnet which is otherwise avoided because of nephrotoxicity [9]. Valganciclovir is the l-valyl ester prodrug of ganciclovir which provides an excellent oral alternative to ganciclovir for the prevention of CMV in transplant recipients [10]. Although valganciclovir is an established drug for CMV disease treatment, this is the first case report of the treatment of CMV skin ulcers with valganciclovir.
In summary, CMV-induced skin ulcers, particularly in axillary regions, have not been previously reported in renal allograft recipients. CMV-induced skin ulcers should be added to the list of manifestations of CMV disease in renal transplant recipients. Skin biopsy may be of help in the prompt diagnosis and treatment of CMV skin ulcers.
Acknowledgments
The authors thank the Council of Science and Technology, UP, India for the research support.
Conflict of interest statement. None declared.
References
- 1.Shaver MJ, Bonsib SM, Abul-ezz S, et al. Renal allograft dysfunction associated with cytomegalovirus infection. Am J Kidney Dis. 1999;34:942–946. doi: 10.1016/S0272-6386(99)70055-7. [DOI] [PubMed] [Google Scholar]
- 2.Kashyap R, Shapiro R, Jordan M, et al. The clinical significance of cytomegaloviral inclusions in the allograft kidney. Transplantation. 1999;67:98–103. doi: 10.1097/00007890-199901150-00017. [DOI] [PubMed] [Google Scholar]
- 3.Lack EE, Chandler FW, Pearson GR. Cytomegalovirus infection. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, editors. Pathology of Infectious Diseases. Connecticut: Appleton and Lange; 1997. pp. 91–99. [Google Scholar]
- 4.Trimarchi H, Casas G, Jordan R, et al. Cytomegalovirus maculopapular eruption in a kidney transplant patient. Transpl Infect Dis. 2001;3:47–50. doi: 10.1034/j.1399-3062.2001.003001047.x. [DOI] [PubMed] [Google Scholar]
- 5.Sterling JC. Virus infections. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. Massachusetts: Blackwell Science Ltd; 2004. pp. 25.1–25.83. [Google Scholar]
- 6.Choi YL, Kim JA, Jang KT, et al. Characteristics of cutaneous cytomegalovirus infection in non-acquired immune deficiency syndrome, immunocompromised patients. Br J Dermatol. 2006;155:977–982. doi: 10.1111/j.1365-2133.2006.07456.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY. Cytomegalovirus infection involving the skin in immunocompromised hosts. A clinicopathologic study. Am J Clin Pathol. 1989;92:96–100. doi: 10.1093/ajcp/92.1.96. [DOI] [PubMed] [Google Scholar]
- 8.Rico JE, Cardona X, Rodelo J, et al. Ureterostomy cytomegalovirus infection presenting as stoma ulceration in a kidney allograft receptor: a case report. Actas Urol Esp. 2008;32:649–652. doi: 10.1016/s0210-4806(08)73903-2. [DOI] [PubMed] [Google Scholar]
- 9.Smith SR, Butterly DW, Alexander BD, et al. Viral Infections after renal transplantation. Am J Kidney Dis. 2001;37:659–676. doi: 10.1016/s0272-6386(01)80114-1. [DOI] [PubMed] [Google Scholar]
- 10.Taber DJ, Ashcraft E, Baillie GM, et al. Valganciclovir prophylaxis in patients at high risk for the development of cytomegalovirus disease. Transpl Infect Dis. 2004;6:101–109. doi: 10.1111/j.1399-3062.2004.00066.x. [DOI] [PubMed] [Google Scholar]