Abstract
Objective. This study aims to highlight the challenges in the diagnosis of hyperparathyroidism (HPT) in patients with advanced chronic kidney disease (CKD).
Methods. In this report, we describe a middle-aged Filipino gentleman with underlying CKD who presented with intractable nausea, vomiting, severe and medically refractory hypercalcaemia and parathyroid hormone (PTH) concentrations in excess of 2400 pg/mL. The underlying pathophysiology as well as the aetiologies and current relevant literature are discussed. We also suggest an appropriate diagnostic approach to identify and promptly treat patients with CKD, HPT and hypercalcaemia.
Results. Evaluation confirmed the presence of a large parathyroid adenoma; HPT and hypercalcaemia resolved rapidly following resection.
Conclusion. This case report is remarkable for its severe hypercalcaemia requiring haemodialysis, large adenoma size, acute-on-chronic kidney injury and markedly elevated PTH concentration in association with primary HPT in CKD.
Keywords: parathyroid adenoma, parathyroid hormone, primary hyperparathyroidism, PTH, tertiary hyperparathyroidism
Introduction
Parathyroid hormone (PTH) is an 84-amino-acid polypeptide that increases serum calcium concentration by increasing calcium reabsorption in the thick ascending limb of the nephron, increasing activated vitamin D (calcitriol) production and indirectly increasing bone reabsorption. Primary hyperparathyroidism (HPT) is a common condition, frequently suspected after routine biochemistry testing reveals hypercalcaemia [1]. Other associated abnormalities include hypophosphataemia and hypomagnesaemia. The cardinal clinical manifestations of primary HPT include nephrolithiasis, bone pain, impaired bone mineralization and non-specific neuropsychiatric symptoms [1,2].
In primary HPT, average intact PTH concentrations are ~120 pg/mL (normal = 10–65 pg/mL) with an average serum calcium concentration <11.5 mg/dL (2.87 mmol/L) [3]. The remaining 10–20% of patients has normal or only minimally elevated PTH concentrations, which are abnormal in the setting of hypercalcaemia. Primary HPT results from dysregulation of PTH secretion, which tends not to be completely autonomous (i.e. partial inhibition occurs with increasing serum calcium concentrations). The incidence is ~4/100 000 [4], with women affected twice as frequently as men. The aetiology of primary HPT includes adenoma, carcinoma, glandular hyperplasia, radiation exposure, multiple endocrine neoplasia syndromes and sporadic genetic mutations [5–7]. Adenoma—usually single but possibly double—accounts for ~94% of the cases of primary HPT. These adenomas are comprised of parathyroid chief cells, are usually encapsulated and may be surrounded by normal parathyroid tissue [8]. The remaining ~6% of primary HPT cases involve multiple gland hyperplasia or parathyroid carcinoma [9].
Secondary HPT is a clinically distinct entity accompanying chronic kidney disease (CKD), typically caused by mild phosphate retention and/or reduced production of 1,25-dihydroxyvitamin D; the latter of which is caused by nutritional vitamin D deficiency and/or impaired 1α-hydroxylation of 25-hydroxyvitamin D due to impaired kidney function. Typically, serum calcium concentrations are normal or slightly low in secondary HPT.
Herein, we present the case of a 55-year-old Filipino man with profoundly elevated PTH and hypercalcaemia requiring haemodialysis who presented with nausea and vomiting and was found to have a parathyroid adenoma.
Case report
A 55-year-old Filipino man with a past medical history of hypertension, hyperlipidaemia, CKD (not taking activated vitamin D derivatives or phosphate binders) and coronary artery disease presented to a hospital in the Philippines with abdominal pain, nausea and post-prandial, non-bloody, non-bilious vomiting. His pre-hospitalization serum creatinine concentration was 3.0 mg/dL. An anterolateral ST segment elevation myocardial infarction was diagnosed, and emergent coronary angiography revealed 70% stenosis of the left anterior descending artery distal to a drug-eluting stent (DES) placed ~2 years earlier. A second DES was successfully placed, and he was discharged home on clopidogrel, aspirin, isosorbide mononitrate, metoprolol, rosuvastatin, imidapril and lansoprazole.
Four weeks prior to admission at our hospital, he was admitted to another hospital in the Philippines with intractable nausea and vomiting, fatigue, generalized weakness, constipation and a 30-lb weight loss. Symptomatic treatment was provided; on physical examination, he was noted to have a palpable left anterior neck mass. Serum chemistries showed markedly elevated serum calcium of 17.28 mg/dL and a serum creatinine of 3.8 mg/dL.
An abdominal ultrasound showed possible renal parenchymal disease, and an abdominal non-contrast computed tomography (CT) was normal except for two non-obstructing, hyper-dense foci (thought to represent stones) in the left kidney. In an attempt to treat hypercalcaemia, normal saline infusion (200 mL/h) and furosemide were begun. A narrow corrected QT (QTc) interval was observed, and he was transferred to the coronary care unit (CCU) for closer monitoring.
On the next day, the serum calcium was 14.29 mg/dL and a narrow QTc interval remained. Haemodialysis was recommended; during catheter insertion, the patient's pulse and blood pressure dropped. Atropine and a dopamine infusion were started, but the patient developed chest pain and non-sustained ventricular tachycardia followed by supraventricular tachycardia, which converted to normal sinus rhythm with intravenous amiodarone. On the same day, a neck ultrasound revealed a 4.5 × 1.2 × 1.2-cm soft tissue mass behind the left lobe of the thyroid as well as a cystic component in the inferior pole with no gross calcification.
The patient's serum calcium was 16.36 mg/dL the following day, with serum phosphorus 4.87 mg/dL. Haemodialysis was attempted, but the catheter malfunctioned. A tunnelled haemodialysis catheter was subsequently placed, but profuse bleeding at the site prohibited immediate use. In the interim, he was treated with additional normal saline, subcutaneous calcitonin and ibandronate, which lowered his serum calcium to 13.77 mg/dL. The serum creatinine increased to 4.99 mg/dL.
Approximately 3 weeks prior to admission at our hospital, he was found to have an elevated PTH of 496.2 pg/mL. Dual-energy X-ray absorptiometry demonstrated osteoporosis (spine T-score −3.28, hip T-score −2.70). A 201Tl/99mTc pertechnetate thyroid scan revealed a minimally enlarged thyroid (4.8 × 2.4 cm right lobe, 4.2 × 2.7 cm left lobe), but suboptimal radioactivity accumulation precluded parenchymal evaluation. Delayed static images of the neck after 99mTc-sestamibi injection showed abnormal radiopharmaceutical accumulation in the region of the superior and inferior left parathyroid glands suggestive of adenoma or hyperplasia. Surgical excision was recommended.
With continued hypotension requiring a continuous dopamine infusion, repeat coronary angiography was performed and showed the recently placed DES to be patent. Cardiology recommended 6 months of clopidogrel therapy before proceeding with parathyroid surgery. He was transferred out of the CCU 2 days later, and cinacalcet was started. The serum calcium initially decreased to 12.49 mg/dL (with serum phosphorus 2.9 mg/dL and serum creatinine 2.5 mg/dL), but increased to 14.69 mg/dL (with serum phosphorus 2.9 mg/dL and serum creatinine 2.6 mg/dL) 2 days later, after which his cinacalcet dose was doubled and another dose of intravenous ibandronate was given. An interdisciplinary care conference concluded that continued medical management of the patient's hypercalcaemia was ineffective. The family requested that the patient be transferred to our hospital for surgery.
In preparation for surgery, a contrast CT scan of the head, neck and thorax showed a 1.8 × 1.4 × 4.5-cm heterogeneously enhancing, well-defined mass lesion spanning the posterior aspect of the left thyroid lobe. Mild compression of the trachea with slight rightward deviation was present. Repeat PTH was 1142.89 pg/mL.
Approximately 1 week prior to admission at our institution, he received another dose of intravenous ibandronate and underwent haemodialysis, which lowered the serum calcium from 16.16 mg/dL (with the pre-dialysis serum creatinine 3.0 mg/dL) to 14.97 mg/dL. The following day, his total serum calcium was 17.28 mg/dL and he developed a fever; vancomycin was started for presumed tunnelled catheter infection. A new permanent haemodialysis catheter was inserted, and he underwent daily haemodialysis, which lowered his total serum calcium to 12.97 mg/dL. He was subsequently transferred.
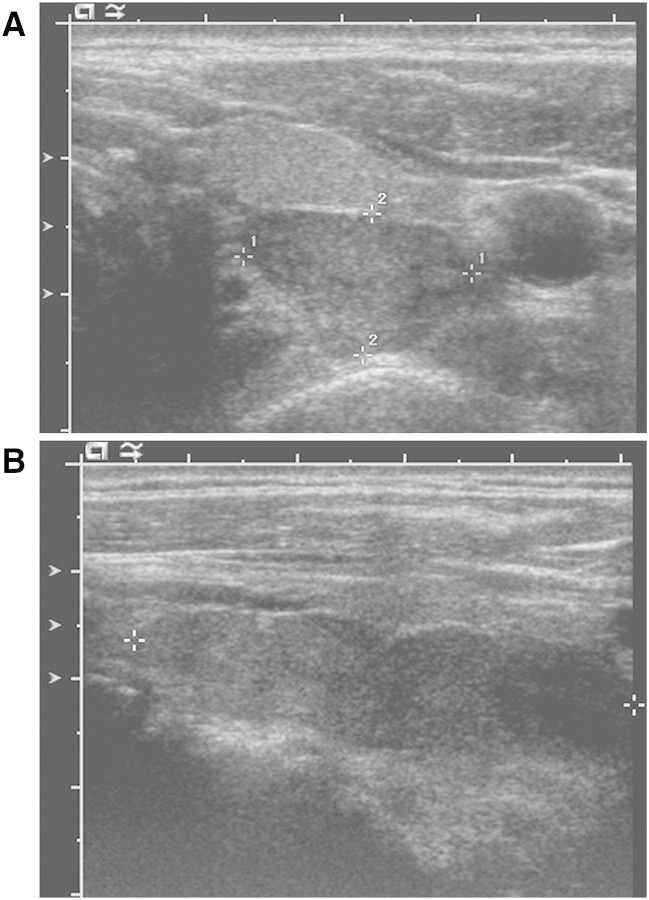
Upon admission to our hospital, the patient's serum calcium was 15.0 mg/dL. The PTH was 1347 pg/mL and increased to 1890 pg/mL the following day. Ultrasound of the thyroid (Figure 1) showed a normal thyroid gland with a 3.7 × 1 × 1.3-cm left lobe and a 4.4 × 1.3 × 1.8-cm right lobe. A large, elongated, hypoechoic, hypervascular mass with internal cystic change measuring 5 × 1.7 × 1 cm was found behind the entire left lobe of the thyroid. Intravenous fluids, furosemide and subcutaneous calcitonin were begun. Serum calcium initially fell to 13.4 mg/dL (with serum phosphorus 4.8 mg/dL) but rose again to 15.7 mg/dL (with serum phosphorus 5.0 mg/dL), and haemodialysis was re-initiated.
Fig. 1.
Ultrasound examination of the thyroid gland. Ultrasound of the thyroid showed a normal thyroid gland with a 3.7 × 1 × 1.3-cm left lobe and a 4.4 × 1.3 × 1.8-cm right lobe that were unremarkable in appearance. A large, elongated, hypoechoic, hypervascular mass with internal cystic change was found behind the entire left lobe of the thyroid. It measured 1.7 × 1 cm in the transverse plane (A, crosshairs 1 and 2, respectively) and ~5 cm in the longitudinal plane (B, crosshairs), suggesting either a massive single parathyroid adenoma or separate adenomas immediately adjacent to each other.
Based on a working diagnosis of parathyroid carcinoma, the patient underwent the planned left parathyroidectomy with concomitant left thyroid lobectomy on the following day. Intraoperative PTH was 2462 pg/mL prior to incision, which decreased to 310 pg/mL immediately after removal and to 144.5 pg/mL within 10 min after removal. Gross pathologic findings included a 4-cm, in greatest dimension, portion of left thyroid with a 3.7 × 1.8 × 1.8-cm solitary abnormal parathyroid gland weighing 2 g. Microscopic examination (Figure 2) revealed a densely cellular proliferation of neoplastic chief cells demonstrating uniformly round, regular nuclei with smooth nuclear membranes, small variably prominent nucleoli and abundant granular cytoplasm. Features (as proposed by Schantz and Castleman in [10]) suspicious for carcinoma—thick tumour capsule, invasion into adjacent tissues, dense sclerosis within tumour and increased mitotic figures (>1/high-power field)—were noticeably absent.
Fig. 2.
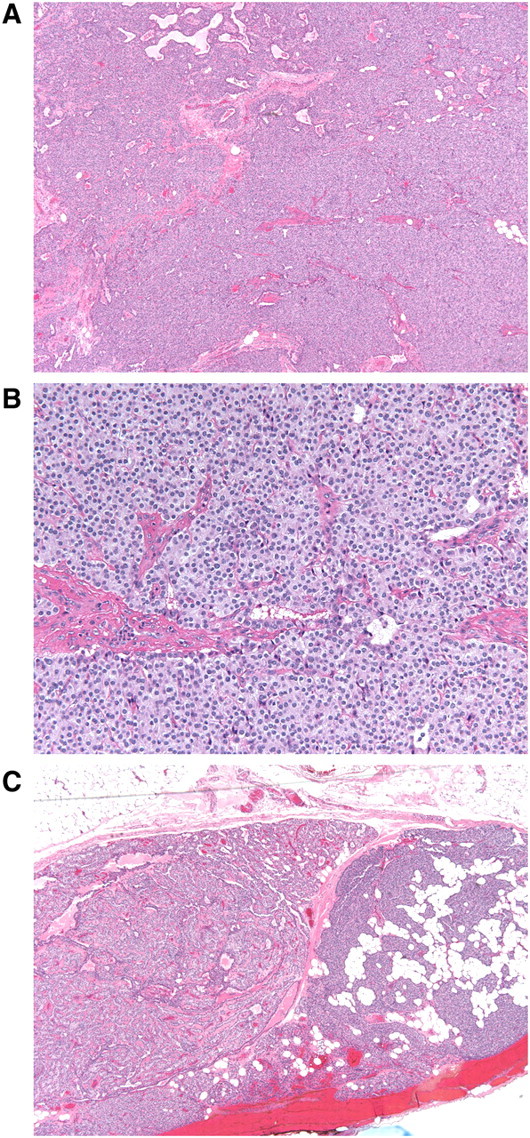
Microscopic examination of excised parathyroid adenoma with normal parathyroid gland. (A) Low-power (×4) image of adenoma showing densely cellular proliferation of chief cells. Note the absence of intervening adipose tissue. (B) High-power (×20) image of adenoma showing a monotonous population of cells with uniformly round, regular, centrally located nuclei, small variably prominent nucleoli and abundant granular cytoplasm. There are no mitotic figures or areas of necrosis. (C) Low-power (×4) image of peripheral aspect of adenoma with adjacent normal parathyroid gland on the right. The adenoma is well circumscribed and shows a trabecular pattern of neoplastic chief cells. Note the interspersed clusters of adipocytes within the adjacent normal parathyroid gland.
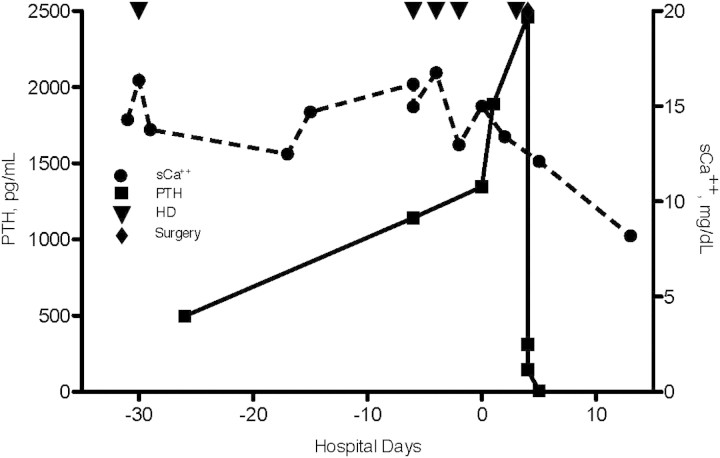
One day after surgery, the PTH declined to 7 pg/mL and the serum calcium declined to 12.1 mg/dL (with serum phosphorus 3.5 mg/dL). His calcium continued to decline until 4 days after discharge with a total serum calcium of 8.2 mg/dL (with serum phosphorus 1.8 mg/dL) (Figure 3). His serum calcium remained stable at 8.7 mg/dL ~6 months after discharge. He required no calcium or phosphorus supplementation.
Fig. 3.
Serum total calcium and PTH concentrations in relation to haemodialysis and parathyroidectomy. Total serum calcium (sCa++) concentrations (black circles, in milligrammes per decilitre) and parathyroid hormone (PTH) concentrations (black squares, in picogrammes per millilitre) are shown over time. Day 0 represents the day of admission at our institution. Haemodialysis (red down-pointing triangles) and parathyroidectomy (blue down-pointing triangles) are indicated.
The serum creatinine at admission to our hospital was 3.1 mg/dL. Ultrasound of the kidneys and bladder with Doppler revealed normal-sized kidneys (11.6 cm right long axis diameter, 11.4 cm left long axis diameter) exhibiting minimal bilateral pelviectasis. A minimally complex cyst with a septa measuring ~13 × 16 × 12 mm in diameter and a second small, simple cyst measuring ~8 × 10 × 9 mm in diameter were found in the left kidney. Numerous, scattered, echogenic foci were seen within the kidneys bilaterally, which may reflect small calcified renal stones. However, there was no evidence for obstruction. The patient has done well with a serum creatinine of 1.5 mg/dL ~6 months after discharge.
Discussion
In this report, we described a case of a middle-aged Filipino man with severe hypercalcaemia—refractory to volume expansion, loop diuretics, bisphosphonates, calcimimetics and haemodialysis—with PTH concentrations in excess of 2400 pg/mL. Diagnostic evaluation confirmed the presence of an extremely large (2 g) parathyroid adenoma. HPT and hypercalcaemia resolved rapidly following adenoma resection. We believe that our patient's findings reflect the most severe clinical manifestations of primary (in contrast to tertiary) HPT ever described in a patient with CKD.
While HPT caused by parathyroid adenoma is common, this case is novel for several reasons. First, severe hypercalcaemia is rare in primary HPT. This degree of hypercalcaemia results only when primary HPT is exceptionally severe (this case) or when tertiary HPT (autonomous PTH hypersecretion after long-standing renal insufficiency) develops. Vasoconstriction induced by severe hypercalcaemia is an important contributing cause of the acute renal failure observed in this patient. The resultant decline in glomerular filtration rate (GFR) most likely accounted for his normal to slightly elevated serum phosphorus concentrations, which are typically low–normal in primary HPT. Indeed, as the patient's hypercalcaemia and kidney function worsened on transfer to our institution, his hyperphosphataemia worsened as well with the serum phosphorus reaching a peak of 5.6 mg/dL on the day of surgery. In the setting of hypercalcaemia where primary or tertiary HPT is suspected, physicians should investigate other aetiologies (e.g. malignancy, thiazide or lithium use, milk–alkali syndrome, hypervitaminosis D and granulomatous disease) in addition to HPT (Table 1). Iatrogenic hypercalcaemia can also result from use of high-dose oral calcium and activated vitamin D derivatives, which are commonly given to patients with end-stage renal disease but are rarely used in patients with lesser degrees of impaired kidney function.
Table 1.
PTH concentrations in various aetiologies of hypercalcaemia
| Disease | PTH concentration |
|---|---|
| Sarcoidosis | Low |
| Multiple myeloma | Low |
| Lymphoma | Low |
| Other malignancy | Low |
| Drugs (e.g. thiazides, lithium) | Low |
| Hypervitaminosis D | Low |
| Thyrotoxicosis | Low |
| Primary HPT | High |
Second, the markedly elevated PTH concentration (>2400 pg/mL) observed in this case is more typical of secondary (or tertiary) HPT. We are confident in the accuracy of these values; at our institution, PTH was measured using a non-competitive immunoassay (Immulite 2000, Siemens Medical Solutions Diagnostics, Newark, DE) with precision documented at 5% CV and linearity verified across the analytically measurable range of 3–2500 pg/ml (R2 = 0.99). In a recent series of 80 patients with primary HPT from adenoma, the highest reported PTH concentration was 2578 pg/mL [11].
Third, the size of this patient's parathyroid adenoma was quite large. In primary HPT, adenoma size is an important determinant of disease severity, but the reason for the large variation (100-fold) in size is unknown. Two recent studies reported normal parathyroid glands weights from 62.4 ± 31.6 mg [12] and from 42.6 ± 20.3 mg [13], respectively. The mean parathyroid adenoma weight was 553.7 ± 520.5 mg [12]. This patient's parathyroid adenoma weighed 2 g, making it significantly larger than most reported parathyroid adenomas. Adenomas weighing more than 60 g have been rarely reported [14,15].
Fourth, this patient suffered acute-on-chronic renal failure, most likely due to hypercalcaemia (peaking at more than 17.0 mg/dL). Serum calcium concentrations from 12.0 to 15.0 mg/dL have been shown to decrease GFR by direct vasoconstriction and natriuresis leading to volume depletion and pre-renal azotemia [16]. Additionally, aquaporin-2 downregulation along with tubulointerstitial injury resulting in impaired osmotic gradient formation may preclude effective urine concentrating mechanisms [17]. In addition to impaired kidney function related to hypercalcaemia-induced vasoconstriction, nephrocalcinosis and possibly acute tubular necrosis (associated with radiocontrast administration and/or hypotension) may have contributed to the acute kidney injury.
Rather than an exceptional case of primary HPT, most of this patient's clinical features are more consistent with either tertiary HPT or parathyroid carcinoma. Parathyroid carcinoma accounted for only 0.74% of more than 22 000 cases of ‘primary HPT’ in a large retrospective study [8]. Total serum calcium concentrations were >14 mg/dL in more than two-thirds of carcinoma cases [9,18]. Nephrolithiasis, nephrocalcinosis and impaired kidney function are found in 32–80% of patients with parathyroid carcinoma compared with 4–18% in benign primary HPT [19]. In this case, there was no evidence of malignancy.
All physicians encountering patients with HPT must be familiar with the multiple aetiologies of hypercalcaemia and understand that ionized calcium is typically maintained in the normal range unless CKD is quite advanced (GFR well below 30 mL/min/1.73 m2) [20]. While uncommon, hypercalcaemia and HPT may exist concurrently from unrelated aetiologies. Malignancies, including multiple myeloma, non-Hodgkin's lymphoma and tumours metastatic to bone can lead to frank or relative hypercalcaemia. However, elevated PTH concentrations typically only occur with concomitant primary HPT (adenoma, carcinoma), and current assays are able to distinguish PTH from rare PTH-related peptide-secreting neoplasms [21]. Thiazide diuretics [22], oral calcium supplementation (including milk–alkali syndrome) and hypervitaminosis D can also result in iatrogenic, PTH-independent hypercalcaemia.
If HPT is confirmed, secondary HPT should be entertained. While most patients with moderate to advanced CKD and evidence of elevated PTH have normal or low serum calcium concentrations, a fraction with low bone turnover may have mild hypercalcaemia. More commonly, iatrogenic hypercalcaemia develops from overzealous management of hyperphosphataemia with calcium-based phosphate binders (calcium carbonate and acetate) and activated vitamin D derivatives. When secondary HPT is refractory to medical therapy and/or PTH fails to suppress in the presence of hypercalcaemia (as in this case), tertiary HPT should be suspected. Subtotal parathyroidectomy should be considered in patients with signs or symptoms referable to HPT, including calcific uremic arteriolopathy (calciphylaxis), fracture, bone pain, myopathy, neuropathy, recalcitrant pruritus or refractory hypertension.
While secondary HPT is the dominant disorder of parathyroid structure and function in patients with CKD, hypercalcaemia in the absence of culprit medications and/or non-suppression of PTH should direct clinicians toward ‘non-secondary’ HPT—either tertiary or, as in this case, primary. The latter may co-exist with secondary HPT and be more subtle and difficult to diagnose. This case illustrates the importance of understanding endogenous and iatrogenic aetiologies of hypercalcaemia and HPT along with an effective diagnostic approach to identify and promptly treat patients with this severe syndrome.
Acknowledgments
We thank Tony Cayco, MD for his assistance in collecting data for this manuscript and James Faix, MD for his assistance with information on the type and performance characteristics of the PTH assay employed at our institution. M.R.L. is supported by the Stanford University School of Medicine Molecular Basis of Medicine scholarly concentration. G.M.C. is supported by K24 DK085446 (mid-career mentoring award) from the National Institutes of Health. The authors declare that the findings presented in this paper have not been published previously.
Conflict of interest statement. G.M.C. has received research support from Amgen, Inc. All other authors have no potential conflict of interest.
Author contributions. M.R.L., J.M.M., L.J.P., J.A.N., A.L.A. and G.M.C. provided patient care. M.R.L., J.M.M. and G.M.C. drafted the manuscript. G.M.C. supervised the project. All authors contributed to, reviewed, edited and approved the final version of the manuscript.
References
- 1.Silverberg SJ, Bilezikian JP. Evaluation and management of primary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:2036–2040. doi: 10.1210/jcem.81.6.8964825. [DOI] [PubMed] [Google Scholar]
- 2.Coker LH, Rorie K, Cantley L, et al. Primary hyperparathyroidism, cognition, and health-related quality of life. Ann Surg. 2005;242:642–650. doi: 10.1097/01.sla.0000186337.83407.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 4.Wermers RA, Khosla S, Atkinson EJ, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21:171–177. doi: 10.1359/JBMR.050910. [DOI] [PubMed] [Google Scholar]
- 5.Krebs LJ, Shattuck TM, Arnold A. HRPT2 mutational analysis of typical sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2005;90:5015–5017. doi: 10.1210/jc.2005-0717. [DOI] [PubMed] [Google Scholar]
- 6.Heppner C, Kester MB, Agarwal SK, et al. Somatic mutation of the MEN1 gene in parathyroid tumours. Nat Genet. 1997;16:375–378. doi: 10.1038/ng0897-375. [DOI] [PubMed] [Google Scholar]
- 7.Pausova Z, Soliman E, Amizuka N, et al. Role of the RET proto-oncogene in sporadic hyperparathyroidism and in hyperparathyroidism of multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 1996;81:2711–2718. doi: 10.1210/jcem.81.7.8675600. [DOI] [PubMed] [Google Scholar]
- 8.Ruda JM, Hollenbeak CS, Stack BC., Jr A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359–372. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Wynne AG, van Heerden J, Carney JA, et al. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197–205. [PubMed] [Google Scholar]
- 10.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–605. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Ozbey N, Erbil Y, Ademoglu E, et al. Correlations between vitamin D status and biochemical/clinical and pathological parameters in primary hyperparathyroidism. World J Surg. 2006;30:321–326. doi: 10.1007/s00268-005-0239-y. [DOI] [PubMed] [Google Scholar]
- 12.Yao K, Singer FR, Roth SI, et al. Weight of normal parathyroid glands in patients with parathyroid adenomas. J Clin Endocrinol Metab. 2004;89:3208–3213. doi: 10.1210/jc.2003-031184. [DOI] [PubMed] [Google Scholar]
- 13.Ghandur-Mnaymneh L, Cassady J, Hajianpour MA, et al. The parathyroid gland in health and disease. Am J Pathol. 1986;125:292–299. [PMC free article] [PubMed] [Google Scholar]
- 14.Power C, Kavanagh D, Hill AD, et al. Unusual presentation of a giant parathyroid adenoma: report of a case. Surg Today. 2005;35:235–237. doi: 10.1007/s00595-004-2902-6. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya A, Endo S, Abe R. Giant adenoma of the parathyroid: report of a case. Surg Today. 1993;23:465–467. doi: 10.1007/BF00309509. [DOI] [PubMed] [Google Scholar]
- 16.Lins LE. Reversible renal failure caused by hypercalcemia. A retrospective study. Acta Med Scand. 1978;203:309–314. doi: 10.1111/j.0954-6820.1978.tb14879.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosen S, Greenfeld Z, Bernheim J, et al. Hypercalcemic nephropathy: chronic disease with predominant medullary inner stripe injury. Kidney Int. 1990;37:1067–1075. doi: 10.1038/ki.1990.87. [DOI] [PubMed] [Google Scholar]
- 18.Hakaim AG, Esselstyn CB., Jr Parathyroid carcinoma: 50-year experience at The Cleveland Clinic Foundation. Cleve Clin J Med. 1993;60:331–335. doi: 10.3949/ccjm.60.4.331. [DOI] [PubMed] [Google Scholar]
- 19.Shane E. Clinical review 122: parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY, Chertow GM. Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol Dial Transplant. 2002;17:1419–1425. doi: 10.1093/ndt/17.8.1419. [DOI] [PubMed] [Google Scholar]
- 21.VanHouten JN, Yu N, Rimm D, et al. Hypercalcemia of malignancy due to ectopic transactivation of the parathyroid hormone gene. J Clin Endocrinol Metab. 2006;91:580–583. doi: 10.1210/jc.2005-2095. [DOI] [PubMed] [Google Scholar]
- 22.Wermers RA, Kearns AE, Jenkins GD, et al. Incidence and clinical spectrum of thiazide-associated hypercalcemia. Am J Med. 2007;120:e9–e15. doi: 10.1016/j.amjmed.2006.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]