Abstract
We report the case of a 69-year-old woman with secondary hyperparathyroidism who underwent maintenance haemodialysis therapy for 17 years and who presented with severe dural calcification and right subdural haematoma. Her dura mater displayed a rock barnacle-like appearance, and cerebral superficial arteries adhered to the sclerotic lesions. On the microscopic observation, calcified tissue with a clear lamellar structure and osteopontin immunoreactivity was observed. Tartrate-resistant acid phosphatase immunoreactive polynucleated giant cells infiltrated around the tissue. Such morphological properties are specific to the calcified tissue formed through a bone formation-like mechanism that is often observed in arterial media in patients with chronic kidney disease.
Keywords: dura matter, ectopic ossification, secondary hyperparathyroidism, subdural haematoma, tartrate-resistant acid phosphatase
Introduction
Soft tissue calcification is one of the major clinical findings of chronic kidney disease-related mineral bone disorder (CKD-MBD), along with abnormalities in laboratory examinations and bone metabolic disorder [1]. However, soft tissue calcification in CKD-MBD appears to be a heterogeneous disease condition. Some of this calcification is a simple calcium and phosphate complex that has been deposited in a chemical manner, while some is produced by cells through a physiological-like mechanism that is observed in bone under physiological conditions. A typical example of such bone-like extraskeletal calcification is often found in the arterial wall, and in fact it has been revealed that the formation mechanism of medial artery calcification is the same as that for bone [2]. Nevertheless, why such bone-like tissue is developed in the arterial wall in CKD patients remains obscure.
Case
A 69-year-old Japanese woman visited our emergency room complaining of sudden headache, nausea and left haemiplegia. She had been undergoing chronic haemodialysis therapy for 17 years due to the advancement of chronic glomerulonephritis. A subtotal parathyroidectomy had been performed due to medical therapy-resistant secondary hyperparathyroidism 15 years previously. The secondary hyperparathyroidism had relapsed in the residual parathyroid gland 2 years previously, and since then 10 μm of maxacalcitol three times a week had been administered. Ticlopidine chloride 100 mg and aspirin 100 mg had also been applied since the symptoms of arterial sclerotic obliterans at her left femoral artery had become overt. Simple X-ray and X-ray computed tomographic examinations revealed obvious calcification around the thoracic aorta, abdominal aorta, bronchial wall and bilateral femoral arteries. No remarkable events had been noticed before the onset of her current symptoms.
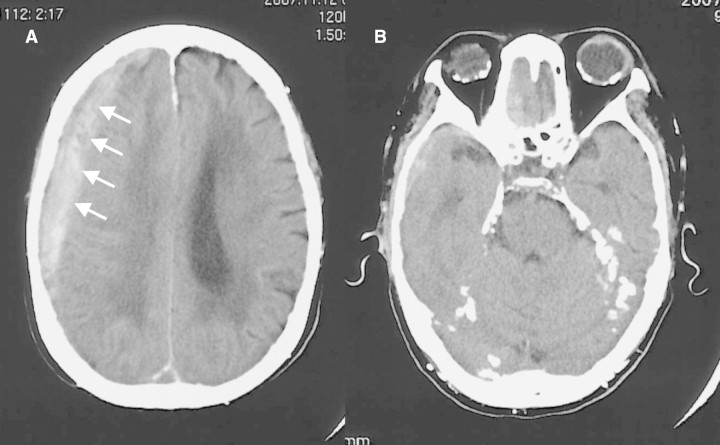
Her height was 145 cm and body weight was 35.8 kg. Her blood pressure was 169/76 mmHg. Despite the left haemiplegia, her consciousness was clear and sensation was intact. The red-eye finding was absent. No remarkable findings were noted at her chest or abdomen. The laboratory results were as follows: haemoglobin 9.6 g/dL, serum creatinine 5.65 mg/dL, urea nitrogen 40.7 mg/dL, sodium 137 mEq/L, potassium 4.0 mEq/L, chloride 108 mEq/L, calcium 10.7 mg/dL, inorganic phosphate 4.1 mg/dL, magnesium 2.0 mg/dL and intact parathyroid hormone 1458 pg/mL. An ultrasonic examination detected a swollen parathyroid gland at her left frontal neck. Cranial computed tomography demonstrated a right subdural haematoma (Figure 1A) in addition to the calcification at the tentorium cerebella and falx cerebri (Figure 1B).
Fig. 1.
Cranial computed tomography (A) Right subdural haematoma (arrows) was confirmed. (B) Overt calcification was noted at the tentorium cerebella and falx cerebri.
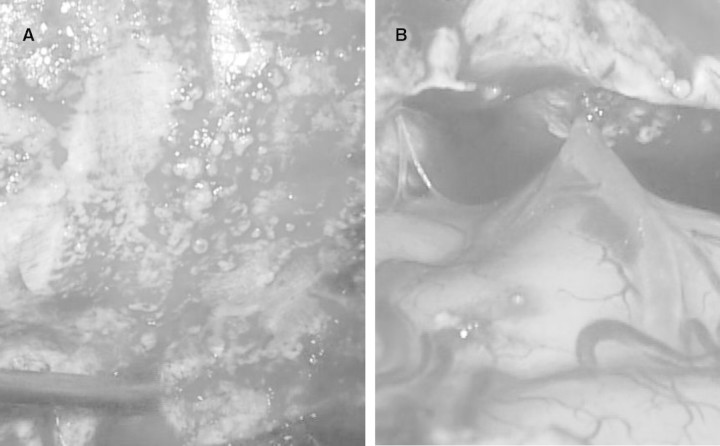
An emergency operation for the subdural haematoma was performed. Sclerotic lesions that looked like many rock barnacles sticking to sea rocks were found in the dura mater (Figure 2A). Adhesions of those sclerotic lesions and cerebral superficial arteries were observed (Figure 2B). Although the artery responsible for the subdural haematoma was not confirmed, the haemorrhage was likely to have been caused in part by such an adhesion. No obvious brain damage was observed.
Fig. 2.
Macroscopic observation of the dura mater. (A) Sclerotic lesions that looked like many rock barnacles sticking to sea rocks were found in her dura mater. (B) Cerebral superficial arteries adhered to such sclerotic lesions.
By histological observation, calcified tissues were found in such sclerotic lesions with a rock barnacle-like appearance, and cells forming a single layer were found on the surface of this tissue (Figure 3A). An obvious lamellar structure was demonstrated in the calcified tissue by a polarization microscopic observation (Figure 3B and C). Osteopontin immunoreactivity was found around the calcified tissue (Figure 4A). Multinucleated giant cells infiltrated near the tissue, and a tartrate-resistant acid phosphatase immunoreactivity was demonstrated in those cells (Figure 4B).
Fig. 3.
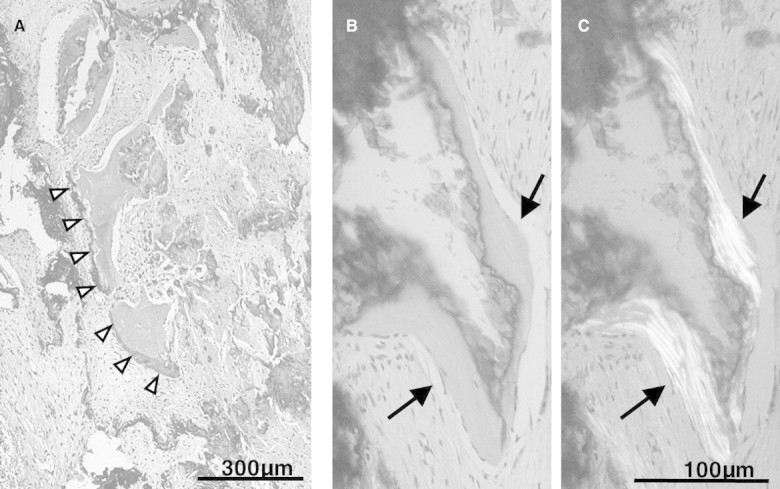
Microscopic findings of the sclerotic dura mater. (A) Calcified tissues were found in such sclerotic lesions with a rock barnacle-like appearance. Cells forming a single layer were found on the surface of this tissue (arrow heads). (B and C) On polarization microscopic observation, a clear lamellar structure was confirmed in the calcified tissue (arrows).
Fig. 4.
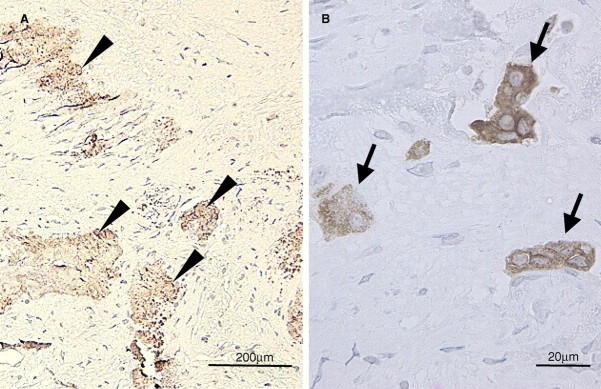
Immunohistochemical findings of the sclerotic dura mater. (A) Osteopontin immunoreactivity was found around the calcified tissue (arrow heads). (B) Tartrate-resistant acid phosphatase immunoreactive multinucleated giant cells infiltrated near the calcified tissue (arrow).
The patient recovered after the emergency operation and was discharged a few days later only presenting lower limb muscle weakness.
Discussion
Although Savazzi [3] has reported that the prevalence of intracranial calcification is 9.6% in dialysis patients, only a few CKD cases with cranial dural calcification have been reported so far [4–8]. Developed secondary hyperparathyroidism and soft tissue calcification in addition to that in the dura mater were observed in these cases. Dorenbeck [7] and Seyahi [8] have reported their cranial computed tomographic findings, which look quite similar to those of the present case except for subdural haemorrhage. The calcification may have been a potential risk for intracranial haemorrhage [4], since the adhesion of superficial arteries was observed (Figure 2B). As far as we are aware, this is the first report of histological and histochemical findings in the calcified dura mater.
Under physiological conditions, a small group of cells called a basic multicellular unit accomplish bone remodelling by ordered group behaviour under yet unknown local and/or systemic inductive factors. Bone formation is one of the steps performed by a group of osteoblasts, and the trace of their regularly repeated bone matrix production remains as a lamellar structure [9]. Quite interestingly, we found an obvious such lamellar structure in the dural calcified tissue. It is, therefore, quite likely that the calcified tissue found in the dura mater was not a mere mineral deposition but cells performing ordered group behaviour to form the calcification. In support of this assumption, the immunoreactivity of osteopontin, which is physiologically found in the bone matrix, was detected around the tissue. Moreover, many tartrate-resistant acid phosphatase immunoreactive multinucleated giant cells that could not be discriminated from osteoclasts also infiltrated around those calcified tissues. Although it is difficult to determine whether or not this tissue is bone, the above histological findings strongly indicate that those calcified tissues were formed and maintained by a group of cells through a mechanism resembling bone metabolism.
In CKD patients, similar bone-like calcified tissue is often found near the medial artery in Mönckeberg-type sclerosis. In Mönckeberg-type medial artery calcification, it is considered that vascular smooth muscle cells transform into osteoblast-like cells and that they form calcified tissue through a mechanism similar to that of physiological bone formation [2]. In fact, Mönckeberg-type medial artery calcification presents properties similar to bone in both morphological and histochemical aspects, which could be called ectopic ossification [10].
Ectopic ossification seems to be frequently found in CKD patients. Although the precise mechanism remains unknown, many investigators have assumed that a systemic abnormality in Ca/Pi/parathyroid/vitamin D metabolism plays a crucial role in its development [11]. Nevertheless, such ectopic ossification has only been found in vascular lesions, especially arterial media in CKD patients. Vascular smooth muscle cells and osteoblasts share their precursor cells, namely mesenchymal stem cells. Therefore, vascular smooth muscle cells would have strong potential to transform into osteoblast-like cells, which may explain why ectopic ossification frequently occurs in medial arteries. However, there may be other potential cells that can transform into osteoblast-like cells. In fact, Couri has reported a non-uraemic case with Mönckeberg's sclerosis in the pharynx and larynx [12].
This CKD case has for the first time demonstrated ectopic ossification in cranial dura mater. Although the precise origin of the dural cells is obscure, dura mater is developed from perineural tube mesenchymal tissue, which some neural crest-derived cells invade. Since neural crest cells are the origin of neurocranial bones, some of the cells in the dura mater have the potential to transform into osteoblast-like cells. In this case, some of the cells in the cranial dura mater displayed an osteoblastic-like function to form bone-like lamellar structured calcified tissue. A so-called salt and pepper appearance is often observed in the cranial X-ray examination in CKD patients with severe secondary hyperparathyroidism. Although the finding has been considered to be due to heterogeneous bone mineral distribution in the cranium, dura mater ossification seems to be another possible explanation for this appearance.
In summary, we encountered a CKD case with secondary hyperparathyroidism and ectopic ossification in the dura mater, which presented a right subdural haematoma. Ectopic ossification in CKD is not a local phenomenon only observed in medial artery. The major factor that induces the transformation of extraskeletal non-osteoblastic cells into osteoblast-like cells in CKD patients would be a systemic factor, with abnormal Ca/Pi/parathyroid/vitamin D metabolism being the most likely candidate.
Conflict of interest statement. None declared.
References
- 1.Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.MaCullough PA, Agrawal V, Danielewicz E, et al. Accelerated atherosclerotic calcification and Mönckeberg's sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585–1598. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- 3.Savazzi GM, Cusmano F, Musini S. Cerebral imaging changes in patients with chronic renal failure treated conservatively or in hemodialysis. Nephron. 2001;89:31–36. doi: 10.1159/000046040. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie WG, Davison AM. Dural calcification: a complication of prolonged periodic haemodialysis. Clin Radiol. 1974;25:349–353. doi: 10.1016/s0009-9260(74)80166-2. [DOI] [PubMed] [Google Scholar]
- 5.Besigye E, Johansen JG, Osnes S. Total dural calcification with secondary hyperparathyoidism: a rare entity. Neuroradiology. 1978;15:107–109. doi: 10.1007/BF00334124. [DOI] [PubMed] [Google Scholar]
- 6.Koroglu M, Chen PS, Oto A, et al. Left atrial, pulmonary vein and dural calcification in a patient with arrhythmia and chronic renal failure. JBR-BTR. 88:78–79. [PubMed] [Google Scholar]
- 7.Dorenbeck U, Leingärtner T, Bretschneider T, et al. Tentorial and dural calcification with tertiary hyperparathyroidism: a rare entity in chronic renal failure. Eur Radiol. 2002;12(Suppl 3):S11–S13. doi: 10.1007/s00330-002-1406-2. [DOI] [PubMed] [Google Scholar]
- 8.Seyahi N, Apaydin S, Sariyar M, et al. Intracranial calcification and tumoural calcinosis during vitamin D therapy. Nephrology. 2004;9:89–93. doi: 10.1111/j.1440-1797.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 9.Roda GA. Introduction to bone biology. Bone. 1992;13(Suppl 1):S3–S6. doi: 10.1016/s8756-3282(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 10.Kazama JJ, Amizuka N, Fukagawa M. Ectopic calcification as abnormal biomineralization. Ther Apher Dial. 2006;10:S34–S38. [Google Scholar]
- 11.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 12.Couri CE, da Silva GA, Martinez JA, et al. Mönckeberg's sclerosis—is the artery the only target of calcification? BMC Cardiovasc Disord. 2005;5:34. doi: 10.1186/1471-2261-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]