Abstract
Cryocrystalglobulinaemia is an extremely rare complication of monoclonal gammopathy. Its presentation has features of both type I and II cryoglobulinaemia. Although peripheral and digital ischaemia is common, visceral ischaemia is rare. When it does occur, it is usually associated with multiple myeloma and has an extremely poor prognosis. We present a case of bilateral renal artery thrombosis associated with cryocrystalglobulinaemia in a patient without myeloma. More unusual, the cryocrystal protein in this case was associated with fibrinogen, which may have led to increased propensity towards thrombosis. Although the patient was unable to recover his kidney function, he remained alive on dialysis 2 years after the incident. The patient did not have any further ischaemic event despite no definitive therapy. This case represents an unusual presentation for this rare disease.
Keywords: cryocrystalglobulinaemia, crystal, monoclonal, renal artery thrombosis
Background
Cryocrystalglobulinaemia is one the rarest manifestations of monoclonal gammopathy characterized by the reversible crystallization of monoclonal immunoglobulins composed of both light and heavy chains below 37°C [1,2]. These crystals can be intracellular, often in plasma cells, or extracellular in various organs and tissues. Initially described in patients with multiple myeloma, cryocrystalglobulinaemia has since been reported with various B-cell lymphoproliferative disorders [3]. Patients typically present with a systemic necrotizing vasculitis manifested by cutaneous purpura and ulcers, mucosal ulcers, erosive polyarthropathy and renal dysfunction [1]. Peripheral ischaemia exacerbated by cold temperature can occur but visceral ischaemia is rare [4,5]. We report a case of cryocrystalglobulinaemia resulting in bilateral renal arterial thrombosis.
Case
A 51-year-old male previously in excellent health developed increasing fatigue and decreased exercise tolerance in 2002. Other symptoms included recurrent sinusitis, transient cough, arthralgias, hives and swollen tender feet. The hives and swelling initially responded to diphenhydramine. He particularly noted that his fatigue worsened with exposure to the cold weather. In September 2003, a purpuric rash appeared on his legs that developed into an ulcer. A skin biopsy revealed a vasculitis with fibrinoid necrosis. In February 2004, the patient was hospitalized for extreme shortness of breath and a serum creatinine (Scr) of 4.1 mg/ dL (362 μmol/L). A renal biopsy was performed.
The renal biopsy (Figure 1) showed eosinophilic immunoglobulin thrombi within many of the arterioles and glomerular capillaries, including the vascular pole of the glomerulus. This material was variably pale to somewhat positive on a PAS stain and was negative on a Congo red stain. Many of the immunoglobulin thrombi were crystalline in shape. Occasional neutrophils were present within the glomerular capillary loops. No true thrombi were noted. The glomeruli did not show mesangial hypercellularity or mesangial matrix expansion, no glomerular basement membrane thickening or duplication was identified and no crescents were present. The biopsy showed focal mild interstitial fibrosis and tubular atrophy. No fibrinoid necrosis of vessels was identified. By immunofluorescence, the immunoglobulin thrombi showed bright staining for IgG, C3, fibrinogen and kappa light chain, lesser staining for C1q and no staining for IgM or lambda light chain.
Fig. 1.
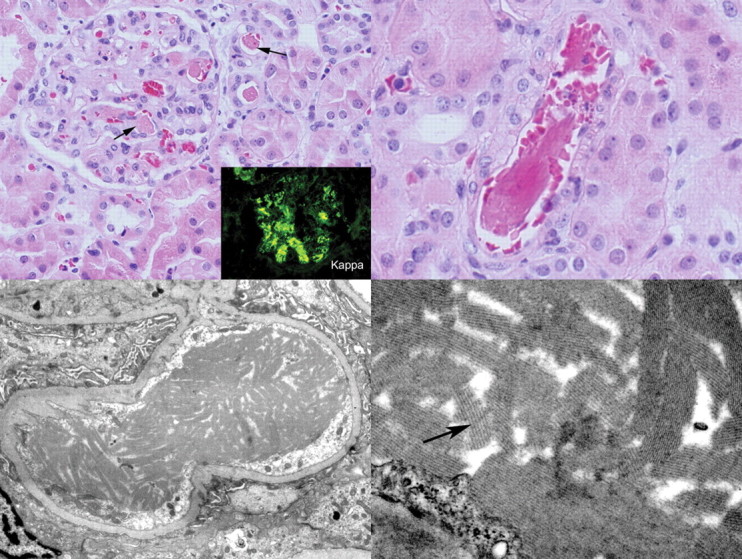
(Top left) Several immunoglobulin thrombi are seen within glomerular capillary lumens and within arteriolar lumens (arrows) (haematoxylin and eosin). By immunofluorescence, these areas stained for kappa light chain (inset) and fibrinogen but not for lambda light chain (immunofluorescence). (Top right) An arteriole contains an immunoglobulin thrombus with a crystalline structure (haematoxylin and eosin). (Bottom left) By electron microscopy, crystalline immunoglobulin thrombi fill a glomerular capillary lumen. (Bottom right) On higher magnification, crystalline immunoglobulin thrombi show a fine periodicity.
Laboratory investigation included testing for cryoglobulins, which were repeatedly negative. Serum protein electrophoresis showed a small monoclonal band in the gamma region, but immunofixation was not performed. A bone marrow biopsy at that time showed no definite evidence of a plasma cell disorder. The peripheral blood count and differential were normal. The serum C3 level was slightly decreased (0.78 g/L, normal range 0.90–1.80 g/L) and the serum C4 level was normal. Tests for HIV, hepatitis B and C infection were negative. Testing for hypercoagulable states was not performed.
The patient's cough and purpura improved significantly with high-dose steroids. Renal function also improved [Scr = 1.9 mg/dL (168 μmol/L)], but he continued to have fatigue. In September 2004, another leg ulcer developed. Another skin biopsy showed leucocytoclastic vasculitis by report. He was treated with azathioprine 50–100 mg/day and prednisone 50 mg/day that was tapered to 5 mg twice a day over 6 months.
In February 2006, the patient presented with rash, fatigue, nausea and vomiting. He had noted dark-coloured urine and quickly became anuric. His blood pressure was 200/100 mmHg and a Scr was 8.2 mg/dL (725 μmol/L). The LDH was elevated at 1253 U/L, but creatinine kinase was normal. Laboratory evaluation including cryoglobulins, ANA, ANCA and ENA were negative, and serum C3 and C4 were normal. Haemoglobin was 14.3 g/dL (143 g/L) with WBC of 8.5 × 109/L, but the platelet count was 12 × 109/L. The peripheral blood smear showed normal red blood cell morphology and no schistocytes.
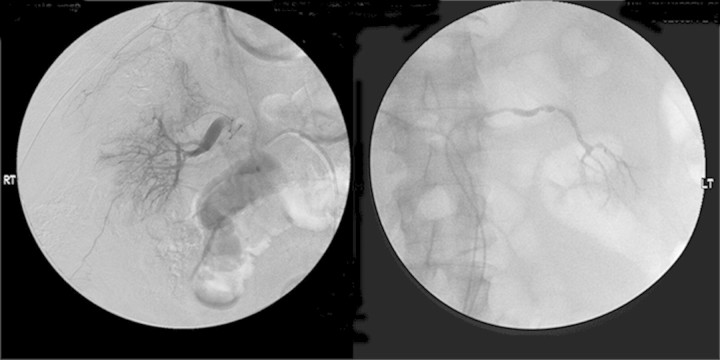
The patient's thrombocytopaenia prevented a renal biopsy at the time and high-dose intravenous methylprednisolone was started empirically. No other causes of acute renal failure, such as initiation of a new drug, were identified. The patient remained anuric, and a renal angiogram (Figure 2) was performed which showed abnormalities of the main, tertiary and quaternary branches with no cortical perfusion. Alteplase was infused for 12 h without any improvement. Thrombophilia testing for factor V Leiden, G20210A prothrombin gene variant and anti-phospholipid antibodies was not performed. He was started on dialysis and remained dialysis dependent. Since starting dialysis, he has not experienced another episode of purpura.
Fig 2.
Renal angiogram: decreased flow bilaterally with abnormalities of the main branch of the left renal artery. Marked abnormalities in the tertiary and quaternary branches with no cortical blush bilaterally. These abnormalities persisted after 12 h of intra-arterial tPA infusion.
In September 2007, patient presented for a second opinion. His total urinary protein level was 133 mg/day in 182 ml of urine a day. Cryoglobulins were again negative along with a test for cryofibrinogenaemia. Serum immunofixation did reveal a monoclonal IgGκ in the serum with an M-spike of 0.4 g/dL (4 g/L). His serum IgA and IgM were suppressed at 35 mg/dL (normal range, 50–400 mg/dL) [0.35 g/L (normal range, 0.05–0.40 g/dL)] and 48 mg/dL (50–300 mg/dL) [0.48 g/L (0.05–0.30 g/L)], respectively. The serum total IgG was normal at 837 mg/dL (normal range, 600–1500 mg/dL (8.37 g/L (6–15 g/L))]. The kappa free light chain was 33.9 mg/L (normal range, 3.3–19.4 mg/L) and lambda was 42.7 mg/L (5.7–26.3 mg/L) with a ratio of 0.79 (0.26–1.65).
The previous renal biopsy was reviewed and additional staining was performed on paraffin-embedded tissue. In addition to positive staining for fibrinogen by immunofluorescence, the immunoglobulin thrombi were also positive on a phosphotungstic acid-haematoxylin (PTAH) stain. No linear staining of glomerular or tubular basement membranes for a monoclonal immunoglobulin was present. Electron microscopy (EM) revealed the immunoglobulin thrombi to be composed of crystalline structures with an organized substructure showing a periodicity of ∼20 nm. In areas of the immunoglobulin thrombi, the endothelial cells appeared reactive, with enlargement of the cells and loss of fenestrations. Widespread podocyte foot process effacement was present. No finely granular deposits suggestive of monoclonal immunoglobulin deposition disease were identified within basement membranes.
Because the renal biopsy showed co-localized monotypic immunoglobulin and fibrinogen, the patient's serum was evaluated by immunoprecipitation for the presence of fibrinogen and was found to be negative. A bone survey was negative. A repeat bone marrow biopsy and aspirate showed minimal involvement by a kappa light chain-restricted plasma cell proliferative disorder based on flow cytometry, with ∼1% plasma cells in the marrow. Bone marrow cytogenetic studies were normal.
Discussion
Cryocrystalglobulinaemia was first described in 1938 in the setting of multiple myeloma [6]. A small number of reports have since been published. Cryocrystalglobulins may precipitate in vivo and in various tissues and organs, particularly in arterial walls causing vasculitis and vessel occlusion [2,3,7]. The deposition of crystals within blood vessels gives rise to the clinical manifestations. Clinically, cryocrystalglobulinaemia usually presents as skin lesions, with recurrent purpura, petechiae and skin ulcers. Leucocytoclastic vasculitis is often seen on skin biopsy. Cryocrystalglobulinaemia may also present as an arthropathy, with crystal deposition in joint fluid [8–10]. Renal and intestinal small vessel involvement, including a clinical picture of systemic vasculitis, has also been described [5,9–12]. Most cases have been associated with multiple myeloma.
Cryocrystalglobulinaemia is similar to type I cryoglobulinaemia in which the precipitated protein forms crystalline structures. Crystals of various shapes, from fusiform to diamond, have been observed. Cryocrystals are usually composed of a monoclonal IgG although a rare light chain only variant has also been described [2–5,8,10,12–15]. There are subtle differences in the clinical presentation of cryocrystalglobulinaemia and type I cryoglobulinaemia. While purpuric rashes, skin ulcers and Raynaud's phenomenon are common to type I cryoglobulinaemia, renal manifestations are rare [16,17]. Cryocrystalglobulinaemia on the other hand also presents with purpura and ulcers, but Raynaud's phenomenon is notably absent. Renal involvement, however, is more common in cryocrystalglobulinaemia.
The true incidence of cryocrystalglobulinaemia is unknown. A review of the literature found ∼50 cases, but it is important to distinguish cryocrystalglobulinaemia and the other immunoglobulin crystalline nephropathies (crystal-storing histiocytosis, light chain Fanconi syndrome or light chain crystal deposition disease) which do not show crystal formation upon cooling [2–5,8,10,12–15,18–22]. In addition, the cutaneous and arthropathic features that are often found in cryocrystalglobulinaemia are absent in these entities. Interestingly, serum tests for cryoglobulins are often negative in patients with cryocrystalglobulinaemia [4,5].
The prognosis of cryocrystalglobulinaemia varies and depends on the presence of multiple myeloma. Patients with so-called essential cryocrystalglobulinaemia (without myeloma) tend to do better with survival often measured in years [2,7,10]. Patients who present with active myeloma do much worse often with a fulminant course [3,5,6,9]. Treatment with chemotherapy has produced mixed results. While some patients respond to steroids alone, others have failed cyclophosphamide and melphalan. Plasma exchange can rapidly improve symptoms, but multiple courses may be needed and durable response must be maintained with effective chemotherapy [4,14]. More recently, improvement in ulcers and renal function was reported with thalidomide and dexamethasone in one patient [7]. Although there is no standard therapy, most authors advocate the importance of early recognition and rapid initiation of effective therapy as the key to better outcomes.
The presentation of bilateral renal arterial occlusion in our patient is unusual, if not unique, in cryocrystalglobulinaemia. First, while renal involvement is common, the complete occlusion of the arterial supply is unusual [4,5,7,10]. Complete vascular occlusion is typically limited to small peripheral vessels although focal visceral ischaemia has been reported [4,5,8]. Precipitation and crystallization are thought to be less likely to occur with the warmer blood in the visceral circulation. Even in the periphery, vascular occlusion had only been reported in patients with advanced myeloma. In those cases, visceral vascular occlusion was a harbinger of the catastrophic peripheral ischaemia and ultimately death [5,8]. Our patient, however, did not have myeloma (1% monoclonal κ restricted plasma cells in the bone marrow) and is still alive 20 months after bilateral renal artery occlusion. The cause of the sudden flare resulting in renal artery occlusion and then disease quiescence despite the persistent presence of the monoclonal IgGκ remains unexplained. One particularly unusual aspect of this case is the co-localization of the monoclonal immunoglobulin thrombi with fibrinogen, as demonstrated by immunofluorescence and by light microscopy by PTAH staining. A somewhat similar case of microvascular thrombosis with a plasma cryoprecipitate composed of fibrinogen and monoclonal IgG-κ has been reported; this patient developed skin ulcers upon exposure to the cold, and laboratory evaluation revealed a monoclonal IgG-κ in the serum and negative tests for cryoglobulins in the serum [23]. We hypothesize that in the current case the IgG-κ paraprotein, unlike other monoclonal immunoglobulins, is particularly thrombogenic, as evidenced by its co-localization with fibrinogen in tissue sections.
As demonstrated by this case, the renal manifestations of monoclonal gammopathy can be vast and diverse. A renal biopsy should be performed in all patients with monoclonal gammopathy and evidence of renal involvement [24]. Early diagnosis is often the key to better outcome.
Conflict of interest statement. None declared.
References
- 1.Rull M. Calcium crystal-associated diseases and miscellaneous crystals. Curr Opin Rheumatol. 1997;9:274–279. doi: 10.1097/00002281-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Dotten DA, Pruzanski W, Olin J, et al. Cryocrystalglobulinemia. Can Med Assoc J. 1976;114:909–912. [PMC free article] [PubMed] [Google Scholar]
- 3.Krause JR, Nitiyanant P, Rabin BS. Cryocrystalglobulinemia in hairy cell leukemia. Cancer. 1978;42:2798–2801. doi: 10.1002/1097-0142(197812)42:6<2798::aid-cncr2820420640>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Ball NJ, Wickert W, Marx LH, et al. Crystalglobulinemia syndrome. A manifestation of multiple myeloma. Cancer. 1993;71:1231–1234. doi: 10.1002/1097-0142(19930215)71:4<1231::aid-cncr2820710410>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Usuda H, Emura I, Naito M. Crystalglobulin-induced vasculopathy accompanying ischemic intestinal lesions of a patient with myeloma. Pathol Int. 1996;46:165–170. doi: 10.1111/j.1440-1827.1996.tb03594.x. [DOI] [PubMed] [Google Scholar]
- 6.von Bonsdorff B, Groth H, Packalen T. On the presence of a high-molecular crystallizable protein in the blood serum in myeloma (abstract) Folia Haematol. 1938;59:184. [Google Scholar]
- 7.Hashimoto R, Toda T, Tsutsumi H, et al. Abnormal N-glycosylation of the immunoglobulin G kappa chain in a multiple myeloma patient with crystalglobulinemia: case report. Int J Hematol. 2007;85:203–206. doi: 10.1532/IJH97.06074. [DOI] [PubMed] [Google Scholar]
- 8.Albert L, Inman R, Gordon DA, et al. Cryocrystalglobulinemia mimicking rheumatoid arthritis and vasculitis. J Rheumatol. 1996;23:1272–1277. [PubMed] [Google Scholar]
- 9.Hasegawa H, Ozawa T, Tada N, et al. Multiple myeloma-associated systemic vasculopathy due to crystalglobulin or polyarteritis nodosa. Arthritis Rheum. 1996;39:330–334. doi: 10.1002/art.1780390224. [DOI] [PubMed] [Google Scholar]
- 10.Papo T, Musset L, Bardin T, et al. Cryocrystalglobulinemia as a cause of systemic vasculopathy and widespread erosive arthropathy. Arthritis Rheum. 1996;39:335–340. doi: 10.1002/art.1780390225. [DOI] [PubMed] [Google Scholar]
- 11.Rengers JU, Touchard G, Decourt C, et al. Heavy and light chain primary structures control IgG3 nephritogenicity in an experimental model for cryocrystalglobulinemia. Blood. 2000;95:3467–3472. [PubMed] [Google Scholar]
- 12.Stone GC, Wall BA, Oppliger IR, et al. A vasculopathy with deposition of lambda light chain crystals. Ann Intern Med. 1989;110:275–278. doi: 10.7326/0003-4819-110-4-275. [DOI] [PubMed] [Google Scholar]
- 13.Cummings FJ, Park CH, Bogaars HA, et al. Successful therapy of crystalcryoglobulinemia: a case report. Med Pediatr Oncol. 1979;7:181–190. doi: 10.1002/mpo.2950070213. [DOI] [PubMed] [Google Scholar]
- 14.Grossman J, Abraham GN, Leddy JP, et al. Crystalglobulinemia. Ann Intern Med. 1972;77:395–400. doi: 10.7326/0003-4819-77-3-395. [DOI] [PubMed] [Google Scholar]
- 15.Kalderon AE, Bogaars HA, Diamond I, et al. Ultrastructure of myeloma cells in a case with crystalcryoglobulinemia. Cancer. 1977;39:1475–1481. doi: 10.1002/1097-0142(197704)39:4<1475::aid-cncr2820390419>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Karras A, Noel LH, Droz D, et al. Renal involvement in monoclonal (type I) cryoglobulinemia: two cases associated with IgG3 kappa cryoglobulin. Am J Kidney Dis. 2002;40:1091–1096. doi: 10.1053/ajkd.2002.36350. [DOI] [PubMed] [Google Scholar]
- 17.Tedeschi A, Barate C, Minola E, et al. Cryoglobulinemia. Blood Rev. 2007;21:183–200. doi: 10.1016/j.blre.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Lukitsch O, Gebhardt KP, Kovary PM. Follicular hyperkeratosis and cryocrystalglobulinemia syndrome. Occurrence in a patient with multiple myeloma. Arch Dermatol. 1985;121:795–798. [PubMed] [Google Scholar]
- 19.Schoengen A, Schreiner T, Anselstetter V, et al. Cryocrystalglobulinemia: pH-dependent precipitation of a monoclonal IgG-kappa-immunoglobulin. Blut. 1989;58:255–260. doi: 10.1007/BF00320915. [DOI] [PubMed] [Google Scholar]
- 20.Gu X, Barrios R, Cartwright J, et al. Light chain crystal deposition as a manifestation of plasma cell dyscrasias: the role of immunoelectron microscopy. Hum Pathol. 2003;34:270–277. doi: 10.1053/hupa.2003.27. [DOI] [PubMed] [Google Scholar]
- 21.Jones D, Bhatia VK, Krausz T, et al. Crystal-storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999;30:1441–1448. doi: 10.1016/s0046-8177(99)90166-1. [DOI] [PubMed] [Google Scholar]
- 22.Nasr SH, Markowitz GS, Stokes MB, et al. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85–96. doi: 10.1111/j.1523-1755.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Bair JS, Wu YC, Lu YC. Cryofibrinogenemia: report of a case. J Formos Med Assoc. 1991;90:99–104. [PubMed] [Google Scholar]
- 24.Markowitz GS. Dysproteinemia and the kidney. Adv Anat Pathol. 2004;11:49–63. doi: 10.1097/00125480-200401000-00005. [DOI] [PubMed] [Google Scholar]