Abstract
Liver–kidney transplantation in patients with primary hyperoxaluria type 1 (PH1) and a high systemic oxalate load is often complicated by oxalate deposition in the renal allograft and loss of renal function. Intensive pre- and post-operative haemodialysis (HD) cannot completely prevent rises in plasma oxalate levels during transplantation because of rebound from saturated oxalate stores. Continuous renal replacement therapy may overcome this problem. In two PH1 patients with extensive oxalate accumulation, we found that intra-operative continuous venovenous haemodiafiltration effectively cleared oxalate and kept oxalate at relatively low levels following preoperative HD.
Keywords: CVVH, dialysis, primary hyperoxaluria, transplantation
Background
Primary hyperoxaluria type 1 (PH1) is an autosomal recessive disorder caused by absence or functional defect of the liver-specific enzyme alanine:glyoxylate aminotransferase (AGT) [1]. AGT deficiency results in excessive production of oxalate, most of which is excreted in the urine as long as renal function is preserved. Many PH1 patients develop renal failure as a result of parenchymal oxalate deposition and renal stone formation. As renal function deteriorates, an increasing fraction of the oxalate production is retained and deposited in renal and extrarenal tissues [2]. Since no form of renal replacement therapy can keep up with the produced oxalate, PH1 patients on dialysis continue to develop systemic oxalate accumulation [1, 2]. Therefore, early transplantation is the treatment of choice. There are different transplantation options in adult PH1 patients with renal failure, e.g. isolated kidney transplantation, sequential liver and kidney transplantation and combined liver–kidney transplantation (LKT). There is no consensus about the best option and an individual strategy is required.
A major problem of renal transplantation in PH1 patients is loss of renal function due to oxalate deposition in the renal allograft. Careful peritransplantation management may reduce the risk of this complication [3]. These measures include intensive haemodialysis (HD) pre-transplantation [3, 4], transplantation from a donor as well-matched as possible to reduce the risk of rejection [3], maintenance of high-volume diuresis post-transplantation [3, 4] and vigorous dialysis in the case of oliguria [3]. Most centres use pre- and post-transplantation HD in PH1 patients who undergo kidney or LKT in order to keep plasma oxalate levels as low as possible. However, oxalate levels may rise during transplantation despite pre-operative HD because of ongoing hepatic oxalate production and rebound from saturated oxalate stores. This is especially relevant in LKT because of the long duration of the procedure. Continuous renal replacement therapy has the advantage that it can be more easily applied for a longer period of time during and after transplantation. We here report the effect of intra-operative continuous venovenous haemodiafiltration (CVVHDF) on oxalate removal in two PH1 patients with extensive systemic oxalate accumulation.
Case reports
Patient 1
This female developed acute anuric renal failure peri-partum at the age of 27 years. Diagnostic workup revealed PH1 as primary cause of renal failure with pre-eclampsia as precipitating factor. PH1 was confirmed by liver biopsy; residual liver AGT activity was 10%. Renal function did not recover. She was treated with pyridoxine. At the time of LKT, she was 38 years old and had been on HD for 11 years. Body weight was 52 kg. In the year preceding transplantation, the HD scheme was five times a week 4 h and pre- and post-HD oxalate levels averaged 130 and 30 μmol/L, respectively. Systemic oxalate accumulation consisted of extensive renal calcinosis, bone disease and peripheral neuropathy. LKT was performed with the organs of an 18-year-old-donor. The liver was placed in an orthotopic position. Next, the kidney was transplanted extraperitoneally. Total operating time was 14 h.
Patient 2
This male patient was diagnosed with PH1 at the age of 6 years when he presented with extensive urolithiasis. The diagnosis was confirmed by liver biopsy; residual AGT activity was 5%. Pyridoxine was started. Urinary oxalate excretion averaged 1500 μmol/24 h. Renal function deteriorated over the years and he started HD at the age of 17 years. At the time of LKT, he was 19 years old and was on HD for 2.5 years. Body weight was 43 kg. In the year preceding transplantation, the HD regimen was four times a week for 5 h and pre- and post-HD oxalate levels averaged 150 and 30 μmol/L, respectively. At the time of LKT, he had no residual renal function. Systemic oxalate accumulation consisted of nephrocalcinosis, bone disease and retinal oxalate deposition. LKT was performed with the organs of a 60-year-old donor. First, orthotopic liver transplantation was performed and, thereafter, extraperitoneal kidney transplantation. Total operating time was 12 h.
HD and CVVHDF settings
In both patients, HD (3 h) was performed pre- and post-transplantation with blood flows and dialysate flows of 350 and 700 mL/min, respectively, using a high-flux cellulose triacetate dialyser with a surface area of 2.1 m2 (Sureflux FB 210U; Nipro, Nissho Corporation, Osaka, Japan) since this dialyser was previously found to have a high oxalate clearance [5].
CVVHDF was started ∼90 min after termination of the pre-transplantation HD session using a 0.9 m2 low-flux AN-69 dialyser (M100; Gambro-Hospal). CVVHDF settings were as follows: blood flow 180 mL/min, pre-dilution substitution fluid flow 2000 mL/h and dialysate flow 2500 mL/h. Bicarbonate-based buffer solutions (HF32 Bic; Dirinco, Rosmalen, The Netherlands) were used for substitution and dialysate fluid. CVVHDF was performed without anticoagulation. No complications occurred during CVVHDF other than clotting of the extracorporeal system. This necessitated exchange of the system three times in Patient 1 and twice in Patient 2. The off-time during clotting episodes was ∼20 min per clotting episode. Oxalate clearance by CVVHDF was assessed every 2 h by taking afferent blood and efferent dialysate samples as described before [5]. Samples were immediately put on ice and processed within 30 min as described previously [6–8] and stored at −20°C for a maximum period of 2 weeks. Oxalate concentrations were determined using isotope dilution mass spectrometry [6–8]. The normal upper plasma reference value in our laboratory is 5.0 μmol/L. The amount of oxalate removed by CVVHDF was estimated by computing the area under the curve depicting the relation between time and dialysate oxalate concentration.
Peritransplantation oxalate levels
As shown in Figure 1A and B, pre-transplantation HD effectively reduced plasma oxalate levels: oxalate fell from 114 to 27 μmol/L in Patient 1 and from 170 to 29 μmol/L in Patient 2. In the 90-min interval between the end of preoperative HD and start of CVVHDF, oxalate levels rose from 27 to 36 μmol/L in Patient 1 and from 29 to 59 μmol/L in Patient 2, indicating rebound. After the start of CVVHDF, plasma oxalate levels gradually fell. In Patient 1, oxalate decreased from 36 μmol/L at the start of CVVHDF to 30 μmol/L just before the patients’ liver was removed. In Patient 2, oxalate decreased from 59 μmol/L at the start of CVVH to 42 μmol/L before removal of the patients’ liver. The time of removal of the patients’ liver (site of the metabolic defect) is relevant since oxalate production stops completely and rises in plasma oxalate levels are exclusively caused by rebound from saturated tissues. After the diseased liver was removed, oxalate continued to decrease gradually. At the end of CVVHDF, plasma oxalate levels were 23 μmol/L in Patient 1 and 26 μmol/L in Patient 2.
Fig. 1.
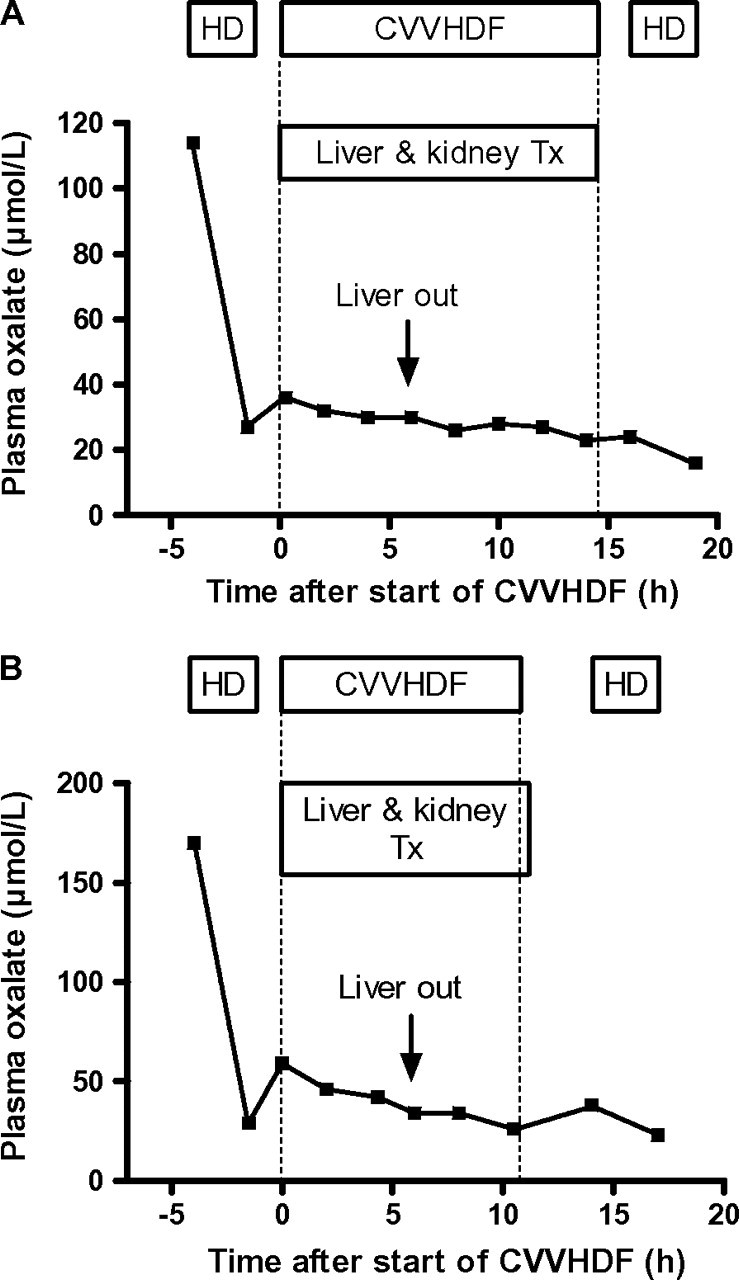
(A) Plasma oxalate levels during pre-operative HD, intra-operative CVVHDF and post-operative HD in Patient 1. The left and the right vertical dotted line indicates the start and the end of CVVHDF treatment, respectively. (B) Plasma oxalate levels during pre-operative HD, intra-operative CVVHDF and post-operative HD in Patient 2. The left and the right vertical dotted line indicate the start and the end of CVVHDF treatment, respectively.
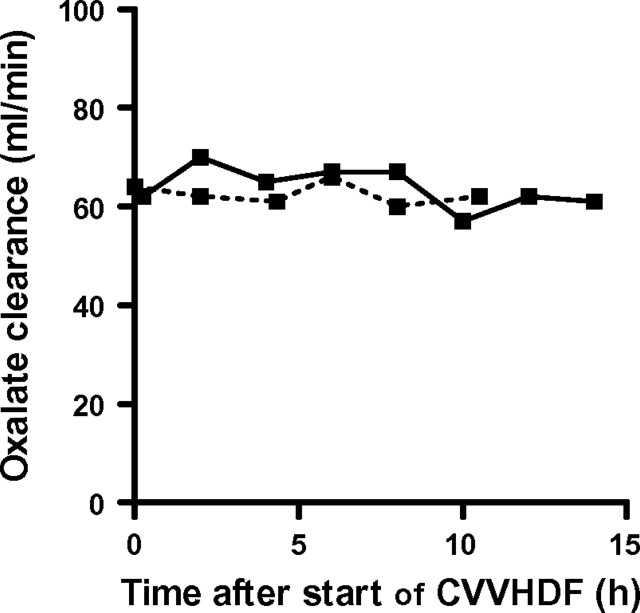
Post-transplantation HD was started 90 min (Patient 1) and 150 min (Patient 2) after cessation of CVVHDF. During the interval between the end of CVVHDF and start of HD, oxalate levels were more or less stable in Patient 1 (23 and 25 μmol/L) but rose in Patient 2 (from 26 to 38 μmol/L), again indicating rebound. Oxalate clearance by CVVHDF was stable over time and averaged (± SD) 64 ± 4 mL/min in Patient 1 and 63 ± 2 mL/min in Patient 2 (Figure 2). The estimated amount of oxalate removed by CVVHDF was 1546 μmol in Patient 1 and 1556 μmol in Patient 2.
Fig. 2.
Oxalate clearance by CVVHDF in Patient 1 (solid line) and Patient 2 (dashed line).
Patient 1 had an uneventful post-operative course with immediate renal transplant function. Patient 2 initially had a diuresis of >60 mL/h but developed hypotension and oliguria at 24 h after the end of LKT. Laparotomy revealed an intra-abdominal haematoma with persistent bleeding from the left hepatic artery. Post-operatively, the patient was haemodynamically stable but oliguria persisted. CVVHDF was restarted using the same settings as with intra-operative CVVHDF except that low-dose heparin was used as an anticoagulant. Post-operative CVVHDF was continued until diuresis and renal function had recovered at Day 9 after LKT in order to keep plasma oxalate levels relatively low. Plasma oxalate levels were measured twice a day during post-operative CVVHDF and averaged 28 μmol/L. Creatinine clearance at 1 year after transplantation is 40 mL/min in Patient 1 and 45 mL/min in Patient 2.
Pharmacokinetic simulations
We predicted the effect of different treatment strategies on the extracorporeal elimination of oxalate by simulation using NONMEM (Icon Development Solutions, Ellicott City, MD). Pharmacokinetic variables were estimated from our two cases using a two-compartment model and a naive-pooled approach. The first compartment was estimated at 34 L and the second compartment was too large for accurate estimation, so we set a large fixed value (10e5). This corresponds to a very large amount of oxalate distributed in the patient’s tissues. Inter-compartmental clearance was estimated at 25 mL/min. For simulations, the LKT was assumed to last 12 h and oxalate clearances by HD and CVVHDF were assumed to be 220 mL/min and 60 mL/min, respectively. Oxalate clearance by the kidney transplant was assumed to start at the end of LKT. Immediate renal transplant function in the first 72 h after LKT was estimated as creatinine clearance of 40 mL/min. Since renal oxalate clearance has been reported to be two to three times the creatinine clearance [9, 10], renal oxalate clearance in case of immediate renal transplant function was estimated as 100 L/min.
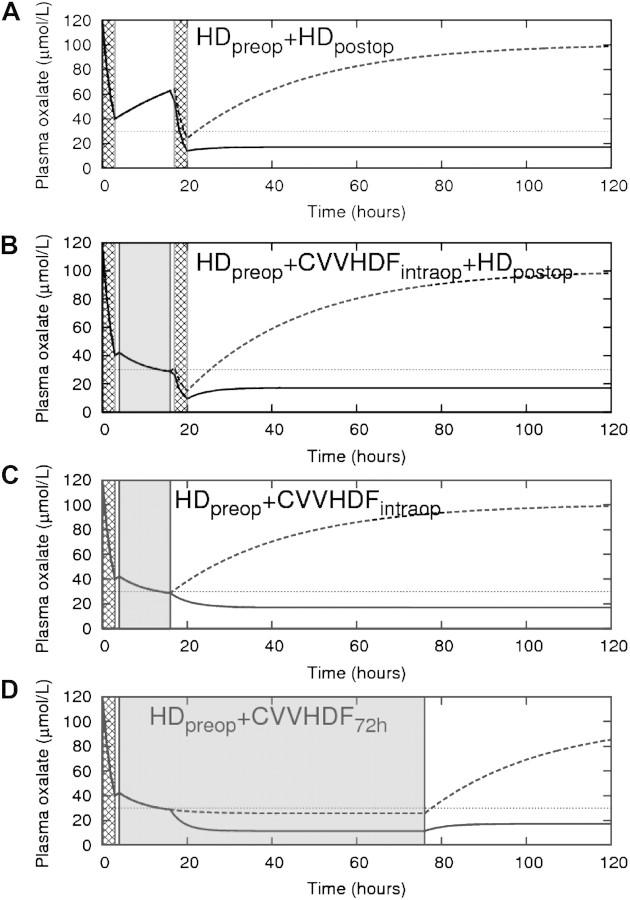
As shown in Figure 3, the combination of pre- and post-operative HD does not prevent an intra-operative rise in plasma oxalate levels (panel a), whereas the addition of intra-operative CVVHDF to this regimen effectively keeps plasma oxalate levels at or below the preoperative post-HD level (panel b). With immediate post-operative renal transplant function, the combination of preoperative HD and intra-operative CVVHDF effectively keeps plasma oxalate at acceptable levels without the need of post-operative HD (panel c). As expected, plasma oxalate levels will rise significantly, independently of the peri-operative treatment strategy, in the case of primary non-function of the renal graft (panel A to C). In this situation, prolonged CVVHDF is capable of keeping oxalate levels <30 μmol/l (panel D).
Fig. 3.
Plasma oxalate levels for different dialysis strategies. The x-axis denotes the time from the start of the preoperative HD session. The hatched areas indicate HD sessions (duration 3 h each); the shaded grey area indicates CVVHDF treatment. In panel (B) and (C), the duration of CVVHDF was 12 h (during LKT); in panel (D), CVVHDF was continued for an additional 72 h after the end of LKT. The solid line indicates plasma oxalate levels in the case of immediate renal transplant function; the dotted line indicates plasma oxalate levels in the case of absent renal transplant function.
Discussion
This report shows that continuous renal replacement therapy during LKT in PH1 patients with renal failure is technically feasible, effectively clears oxalate and completely prevents the post-dialysis rebound of plasma oxalate levels. Although the option of intra-operative continuous renal replacement therapy has been suggested before [4], this report is the first detailed description of its efficacy. In our opinion, this approach is specifically relevant for patients with high systemic oxalate loads (as was the case in both patients) because of the expected rebound of oxalate levels during transplantation.
CVVHDF achieved oxalate clearances of ∼60 mL/min which is lower than with HD. We previously found that, using the same regimen as in both patients in this report, HD achieved oxalate clearances of 220 ± 12 mL/min [5]. In our opinion, high-efficiency HD just before transplantation is crucial in order to effectively and rapidly lower plasma oxalate levels. Thereafter, CVVHDF is capable of keeping oxalate concentrations at the immediate post-HD level. Post-operatively, we did not yet have the results of the oxalate levels and, therefore, we performed HD immediately post-operatively as is our usual routine in patients with high systemic oxalate loads irrespective of post-operative renal allograft function. Now that we have observed that the combination of pre-operative HD and intra-operative CVVHDF effectively keeps plasma oxalate at relatively low levels, it seems plausible to skip the post-operative HD session in the case of immediate renal transplant function. In the case of delayed renal graft function, prolonged CVVHDF may be a good alternative for intermittent HD. However, the optimal duration of post-operative CVVHDF has to be determined in future studies before this approach can be recommended.
Patient 1 was on dialysis for 11 years before she was transplanted. Such a long interval is not recommended since it is associated with ongoing tissue oxalate deposition and carries a greater risk of post-transplantation renal failure due to the high oxalate burden. Unfortunately, there were various reasons for this long interval to transplantation, including significant co-morbidity and anti-human leucocyte antigen antibodies.
The only side effect of CVVHDF was clotting of the extracorporeal system. Clotting occurred thrice in patient 1 and twice in patient 2 and contributed to the blood transfusion requirements during the operation. We chose not to use any anticoagulation because of the high risk of bleeding during liver surgery. Locoregional anticoagulation with citrate should be avoided in the anhepatic phase but may well be a good option during the remainder of the operation. Nevertheless, even without anticoagulation, the total off-time as a result of clotting of the extracorporeal system was relatively low. Other potential side effects of CVVHDF include lowering of body temperature and electrolyte and acid–base shifts. It follows that body temperature, electrolytes and the acid–base balance should be checked at regular intervals during the procedure.
In conclusion, intra-operative CVVHDF during LKT is feasible and completely prevents the post-HD rebound of plasma oxalate levels. Whether this approach improves the long-term renal allograft outcome remains to be studied.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Danpure CJ. Molecular aetiology of primary hyperoxaluria type 1. Nephron Exp Nephrol. 2004;98:e39. doi: 10.1159/000080254. [DOI] [PubMed] [Google Scholar]
- 2.Hoppe B, Graf D, Offner G, et al. Oxalate elimination via hemodialysis or peritoneal dialysis in children with chronic renal failure. Pediatr Nephrol. 1996;10:488. doi: 10.1007/s004670050145. [DOI] [PubMed] [Google Scholar]
- 3.Scheinman JI, Najarian JS, Mauer SM. Successful strategies for renal transplantation in primary oxalosis. Kidney Int. 1984;25:804. doi: 10.1038/ki.1984.93. [DOI] [PubMed] [Google Scholar]
- 4.Monico CG, Milliner DS. Combined liver-kidney and kidney-alone transplantation in primary hyperoxaluria. Liver Transpl. 2001;7:954. doi: 10.1053/jlts.2001.28741. [DOI] [PubMed] [Google Scholar]
- 5.Franssen CFM. Oxalate clearance by haemodialysis—a comparison of seven dialysers. Nephrol Dial Transplant. 2005;20:1916. doi: 10.1093/ndt/gfh971. [DOI] [PubMed] [Google Scholar]
- 6.Wolthers BG, Hayer M. The determination of oxalic acid in plasma and urine by means of capillary gas chromatography. Clin Chim Acta. 1982;120:87. doi: 10.1016/0009-8981(82)90080-8. [DOI] [PubMed] [Google Scholar]
- 7.Wolthers BG, Meijer S, Tepper T, et al. The determination of oxalate in haemodialysate and plasma: a means to detect and study ‘hyperoxaluria’ in haemodialysed patients. Clin Sci. 1986;71:41. doi: 10.1042/cs0710041. [DOI] [PubMed] [Google Scholar]
- 8.Koolstra W, Wolthers BG, Hayer M, et al. Development of a reference method for determining urinary oxalate by means of isotope dilution-mass spectrometry (ID-MS) and its usefulness in testing assays for urinary oxalate. Clin Chim Acta. 1987;170:227. doi: 10.1016/0009-8981(87)90132-x. [DOI] [PubMed] [Google Scholar]
- 9.Prenen JA, Dorhout Mees EJ, Boer P. Plasma oxalate concentration and oxalate distribution volume in patients with normal and decreased renal function. Eur J Clin Invest. 1985;15:45–49. doi: 10.1111/j.1365-2362.1985.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 10.Kasidas GP, Nemat S, Rose GA. Plasma oxalate and creatinine and oxalate/creatinine clearance ratios in normal subjects and in primary hyperoxaluria. Evidence for renal hyperoxaluria. Clin Chim Acta. 1990;191:67–77. doi: 10.1016/0009-8981(90)90059-2. [DOI] [PubMed] [Google Scholar]