Abstract
AA amyloidosis, or secondary amyloidosis, is a rare but serious complication of chronic inflammatory diseases. Chronic inflammatory arthritis is the commonest cause of AA amyloidosis and, when the latter appears, treatment can be frustrating. Deposition of fibrils, derived from circulating acute-phase reactant serum amyloid A protein (SAA), in the kidneys can lead to proteinuria and progressive loss of renal function. We describe the case of a 14-year-old female with systemic juvenile idiopathic arthritis who developed nephrotic syndrome secondary to AA amyloidosis; while she was unresponsive to all measures, including anti-tumour necrosis factor therapy, treatment with tocilizumab, an anti-human interleukin-6 receptor antibody, immediately normalized the SAA and reversed the nephrotic syndrome. We discuss this new therapeutic approach.
Keywords: IL-6 receptor antibody, nephrotic syndrome treatment, secondary amyloidosis, systemic juvenile idiopathic arthritis, tocilizumab
Background
Systemic juvenile idiopathic arthritis (sJIA) is a heterogeneous form of arthritis in childhood and represents 10–20% of all juvenile idiopathic arthritis. It is characterized by the association of chronic arthritis with systemic features, such as spiking fever, macular erythematous rash, serositis, hepatosplenomegaly and lymphadenopathy with prominent laboratory evidence of systemic inflammation. Aetiology of sJIA is largely unknown; however, recent studies suggest that interleukin-6 (IL-6) plays an important role in this entity. Medical treatment of patients who are at the more severe end of the disease spectrum is unsatisfactory. Moreover, up to 30% of patients will still have active disease after 10 years and morbidity within this group is high [1].
Amyloidosis, the major cause of death in sJIA patients during the 1960s and 1970s, only affects the most serious cases. Chlorambucil, and more recently anti-tumour necrosis factor (TNF) agents, have been proposed as AA amyloidosis treatment in sJIA although only some 30% of patients respond to anti-TNF therapy [1, 2].
We report a patient with sJIA and nephrotic syndrome due to AA amyloidosis successfully treated with an anti-interleukin-6 receptor (anti-IL-6R) antibody, tocilizumab. Blocking the transmembrane signalling of IL-6, resulted in complete normalization of serum amyloid A protein (SAA) levels and resolution of the nephrotic syndrome.
Case report
A 14-year-old female was admitted in November 2007, for progressive generalized oedema. At the age of 2, she was diagnosed with sJIA. Since the early diagnosis, the disease was always very aggressive. She had previously been evaluated at multiple institutions and, despite different treatments including biological treatment with anti-TNF, no proper control of her disease was achieved. Current treatment included methotrexate, prednisolone and non-steroidal anti-inflammatory drugs.
Physical examination revealed a blood pressure of 110/70 mmHg, cushingoid appearance, growth impairment, marked peripheral oedema and joint pain in both hands and knees. Laboratory studies showed normal haemoglobin and leucocyte levels, a platelet count of 605 000/mm3, erythrocyte sedimentation rate (ESR) 49 mm, C-reactive protein (CRP) 1.8 mg/dL (normal value 0.1–0.6 mg/dL), serum creatinine 0.85 mg/dL (64.8 μmol/L), cholesterol 282 mg/dL (7.29 mmol/L), total protein 5.4 g/dL and albumin 2.4 g/dL. SAA levels were 163 mg/L (normal value < 10 mg/L). Antinuclear and anti-cytoplasmatic antibodies were negative. A proteinuria of 7 g/24 h was found, and the urine sediment showed microhaematuria as the only relevant finding. Renal ultrasound showed normal kidneys. Renal biopsy (Figure 1) showed both glomerular and vascular amyloid deposition. Immunohistochemistry was consistent with AA amyloid. Unfortunately, electron microscopy could not be performed at our institution.
Fig. 1.
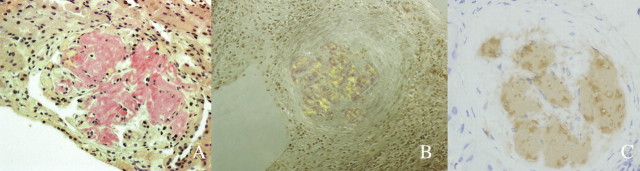
(A) Amyloid with glomerulus infiltrated by homogeneous acellular material replacing normal structures (Congo red stain ×400). (B) Apple green birefringence under polarized light, magnification ×200. (C) Immunostaining for amyloid A protein is positive in the distribution of the amyloid deposits, indicating AA amyloidosis (Amyloid A, clone mc1; hematoxylin, ×400).
Due to the disease resistance to previous treatments and to the new onset of nephrotic syndrome triggered by AA amyloid, treatment with inhibitor of IL-6 receptor, tocilizumab (RoActemra®; Roche Pharma), was started. Prior to tocilizumab treatment, informed consent was obtained. Tocilizumab at a dose of 8 mg/kg was administered intravenously at 2-week intervals.
After 1 month of treatment, the nephrotic syndrome resolved, with a drastic reduction of proteinuria (0.8 g/24 h) and normalization of serum protein and albumin. The oedema disappeared and her functional ability score improved. Her SAA concentration immediately decreased to the normal range of 6.6 mg/dL, and it has remained at this level. Because of this clinical improvement, her family refused a new renal biopsy. It was possible to taper the patient’s treatment with prednisolone and we could also stop the rest of the medication.
A year later, the patient remains asymptomatic with normal renal function (serum creatinine 0.95 mg/dL), absence of proteinuria (0.06 g/24 h), ESR 13 mm, CRP 0.09 mg/dL, total protein 7.1 g/dL and albumin 4.2 g/dL, SAA levels 6 mg/L and the arthritis is in remission. At the moment, she remains on treatment with tocilizumab in a monotherapy administration at 4 mg/kg/2 weeks.
Discussion
Reactive systemic AA amyloidosis can complicate chronic inflammatory disorders that are associated with a sustained acute-phase response. AA amyloid fibrils are derived from the acute-phase reactant SAA protein through a process of cleavage, misfolding and aggregation into a highly ordered abnormal β-sheet conformation [3]. Currently, the most frequent cause of AA amyloidois is inflammatory arthritis. AA amyloidosis occurs in susceptible individuals who have persistent disease activity, which is refractory to all modalities of treatment. The main feature of amyloidosis at diagnosis is renal dysfunction, and the most common finding is proteinuria in the nephrotic range, which does not necessarily develop at the onset [4].
Recent studies have shown that IL-6 in combination with other pro-inflammatory cytokines plays a critical role in the synergic induction of human SAA genes and that inhibition of IL-6 is critical to achieving complete suppression of SAA production [5]. On the other hand, it has been demonstrated that IL-6 is a major contributor to the pathophysiology of inflammatory arthritis, including rheumatoid arthritis (RA) and sJIA. Because of the central role played by IL-6 in a number of manifestations of these inflammatory diseases, IL-6 is a candidate target for therapeutic inhibition as a novel approach to treatment of RA, sJIA and perhaps other related rheumatic diseases [6].
Tocilizumab is a humanized anti-human IL-6 receptor antibody that binds to the soluble and membrane-bound IL-6 receptor, and it is capable of almost completely blocking the transmembrane signalling of IL-6. Furthermore, it has been shown that tocilizumab has an excellent ability to suppress serum amyloid A levels and could therefore be an important therapeutic strategy in amyloid A amyloidosis secondary to rheumatic diseases [6, 7]. Clinical trials of tocilizumab have shown the effectiveness in monotherapy for both RA and sJIA [8, 9]. Three cases (one patient with very active JIA and two patients with RA) of successful treatment with tocilizumab for gastrointestinal AA amyloidosis have been previously reported. One of them had associated renal amyloidosis, although no nephrotic syndrome developed [10].
Our patient had been markedly resistant to treatment with prednisolone, immunesupressive agents and anti-TNF biological modifiers while her condition became complicated with nephrotic syndrome secondary to AA amyloidosis. Toziluzumab treatment not only rapidly reversed the nephrotic syndrome and reduced inflammatory parameters like SAA and CRP but also allowed the discontinuation of all other medications. One year later, the patient’s functional capacity has improved dramatically, and she remains in remission of the nephrotic syndrome. No adverse effects have appeared during the follow-up.
Because a second renal biopsy was not performed, it is unknown if, after a long period of time, there are still deposits of amyloid since amyloid may persist for years despite remission of the nephrotic syndrome [11].
It is unlikely that regression of the existing amyloid can occur quickly. More likely alternatives are that SAA or precursors to mature amyloid fibrils have a functional effect that results in proteinuria or that new amyloid deposits have a greater proteinuric effect than existing deposits already incorporated into the tissue.
Moreover, it is not ruled out that inhibition of IL-6 in the kidneys, modifying the local inflammatory response, could also play an important role in the disappearance of the proteinuria [12, 13].
Treatment with tocilizumab may therefore represent an important therapeutic strategy for AA amyloidosis secondary to rheumatic diseases that otherwise do not respond to other therapies.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol. 2006;2:28–34. doi: 10.1038/ncprheum0084. [DOI] [PubMed] [Google Scholar]
- 2.Kimura Y, Pinho P, Walco G, et al. Etanercept treatment in patients with refractory systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2005;32:935–942. [PubMed] [Google Scholar]
- 3.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 4.Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 5.Hagihara K, Nishikawa T, Isobe T, et al. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun. 2004;314:363–369. doi: 10.1016/j.bbrc.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 6.Lipsky PE. Interleukin-6 and rheumatic diseases. Arthritis Res Ther. 2006;8(Suppl 2):S4. doi: 10.1186/ar1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biologics. 2008;2:75–82. doi: 10.2147/btt.s1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 10.Okuda Y, Takasugi K. Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum. 2006;54:2997–3000. doi: 10.1002/art.22118. [DOI] [PubMed] [Google Scholar]
- 11.Méry JP. Spontaneous remission of nephrotic syndrome in amyloidosis. Br Med J. 1978;2:202–203. doi: 10.1136/bmj.2.6131.202-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mäkelä S, Mustonen J, Ala-Houhala I, et al. Urinary excretion of interleukin-6 correlates with proteinuria in acute Puumala hantavirus-induced nephritis. Am J Kidney Dis. 2004;43:809–816. doi: 10.1053/j.ajkd.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]


