Abstract
Background and objectives. The mesangial deposition of IgA is rarely described with proliferative glomerulonephritis associated with Staphylococcus infection. Recently, this association has been increasingly recognized possibly due to the increased rate of Staphylococcus infection.
Design setting, participants and measurements. We report two cases of methicillin-sensitive Staphylococcus aureus bacteremia associated with acute proliferative glomerulonephritis with dominant mesangial deposit of IgA. We searched MEDLINE (1960–2009) for similar reports. We pooled individual patient data and reported descriptive statistics of all published cases.
Results. Forty-six cases were included in the final analysis. The mean age of presentation was 59, with a male predominance (84%). Clinical presentation was notable for rapidly progressive glomerulonephritis with nephrotic-range proteinuria and normal complement levels in 52 and 72%, respectively. Methicillin-resistant S. aureus (68%) was the most common pathogen isolated with a latent period ranging from 1 to 16 weeks. Diffuse mesangial proliferation was commonly found with crescentic lesions noted in 35% of the cases. Antimicrobial treatment was associated with renal recovery in 58% of the cases. Need for renal replacement therapy was significantly associated with pre-existing diabetes, hypertension and interstitial fibrosis seen on kidney biopsy.
Conclusions. IgA-dominant post-Staphylococcus glomerulonephritis is a rare clinical entity with certain unique clinical and morphologic features. It is difficult to differentiate from primary IgA nephropathy in cases where the infection is not apparent. An acute onset of rapidly progressive glomerulonephritis, with normal complement levels and deposition of mesangial IgA in an elderly patient should raise suspicion for this rare form of glomerulonephritis.
Keywords: acute glomerulonephritis, complement, IgA, Staphylococcus
Background
The mesangial deposition of IgA immune complex is not typically described with acute postinfectious glomerulonephritis (APIGN). Its presence in renal biopsy is usually indicative of IgA nephropathy or Henoch–Schönlein purpura. However, a recent review of 86 cases of APIGN showed that glomerular IgA staining was detected in 44% of cases and Staphylococcus was the culprit infection in 23% of all cases (1). Classically, Staphylococcus infection-related glomerulonephritis is associated with glomerular immune complex deposits, which contain complement (mainly C3), IgG and sometimes IgM. A distinct form of Staphylococcus-associated glomerulonephritis characterized by IgA-dominant or codominant glomerular deposits has been increasingly recognized. We hereby report two such cases as well as a review of the 46 cases reported in the literature.
Case 1
Clinical history and initial laboratory data
A 73-year-old white man with a history of colon cancer, diabetes mellitus type 2, hypertension and coronary artery disease was transferred to our hospital for management of recurrent right pleural effusion after he underwent a resection of liver metastasis. At the outside hospital, culture of the pleural fluid yielded no growth, but his blood cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA).
On physical examination, the patient was afebrile, hemodynamically stable with anasarca and bilateral crackles on lung auscultation. There was no skin rash. His basic metabolic profile was normal. A computed tomography scan of the chest and abdomen showed persistence of the right pleural effusion and subnephric fluid collection. He was continued on intravenous (i.v.) vancomycin and underwent ultrasound-guided drainage of both collections. After 2 weeks, the patient developed acute kidney injury with a rise in creatinine to 3.6 mg/dL, decreased urine output, macroscopic hematuria and nephrotic-range proteinuria (13 g/24 h). He did not have other features of nephrotic syndrome such as hypoalbuminemia and hypercholesterolemia. Urine analysis showed dysmorphic red blood cells (RBCs) without any cellular casts. Serology for viral hepatitis, HIV, antinuclear antibodies (ANAs), Anti-neutrophil cytoplasmic antibodies (ANCAs), C3, C4 and cryoglobulin were normal. Urine and serum protein electrophoresis were normal. The patient underwent a kidney biopsy on hospital Day 20 and was started on intermittent hemodialysis (IHD).
Kidney biopsy
Seventeen glomeruli were present, 10 of which showed both endocapillary and mesangial hypercellularity with leukocyte infiltration. One glomerulus had a cellular crescent, four glomeruli had segmental hypercellularity, two were globally sclerotic, and one was unremarkable. Neither fibrinoid necrosis nor fibrous adhesions were seen. There was a mild degree (10%) of tubular atrophy and interstitial fibrosis. A mild chronic inflammatory area composed of lymphocytes and occasional plasma cells was seen in the interstitium (Figure 1A). Immunofluorescence revealed IgA and C3c deposition in a 3+ granular pattern, predominantly mesangial but with occasional segmental capillary loop staining. Kappa and lambda were 1+ in similar distribution. IgG, IgM, C1q and albumin were negative (Figure 1B). On ultrastructural examination, the glomerular basement membrane was mildly thickened (mean of 0.54 μ with an adult normal range being 0.23–0.48 μ). Mesangial deposits were predominant, with occasional subendothelial deposits. No subepithelial humps were present. Extensive epithelial foot process effacement was evident (Figure 1C). A diagnosis of diffuse proliferative glomerulonephritis with dominant mesangial deposits of IgA was made.
Fig. 1.
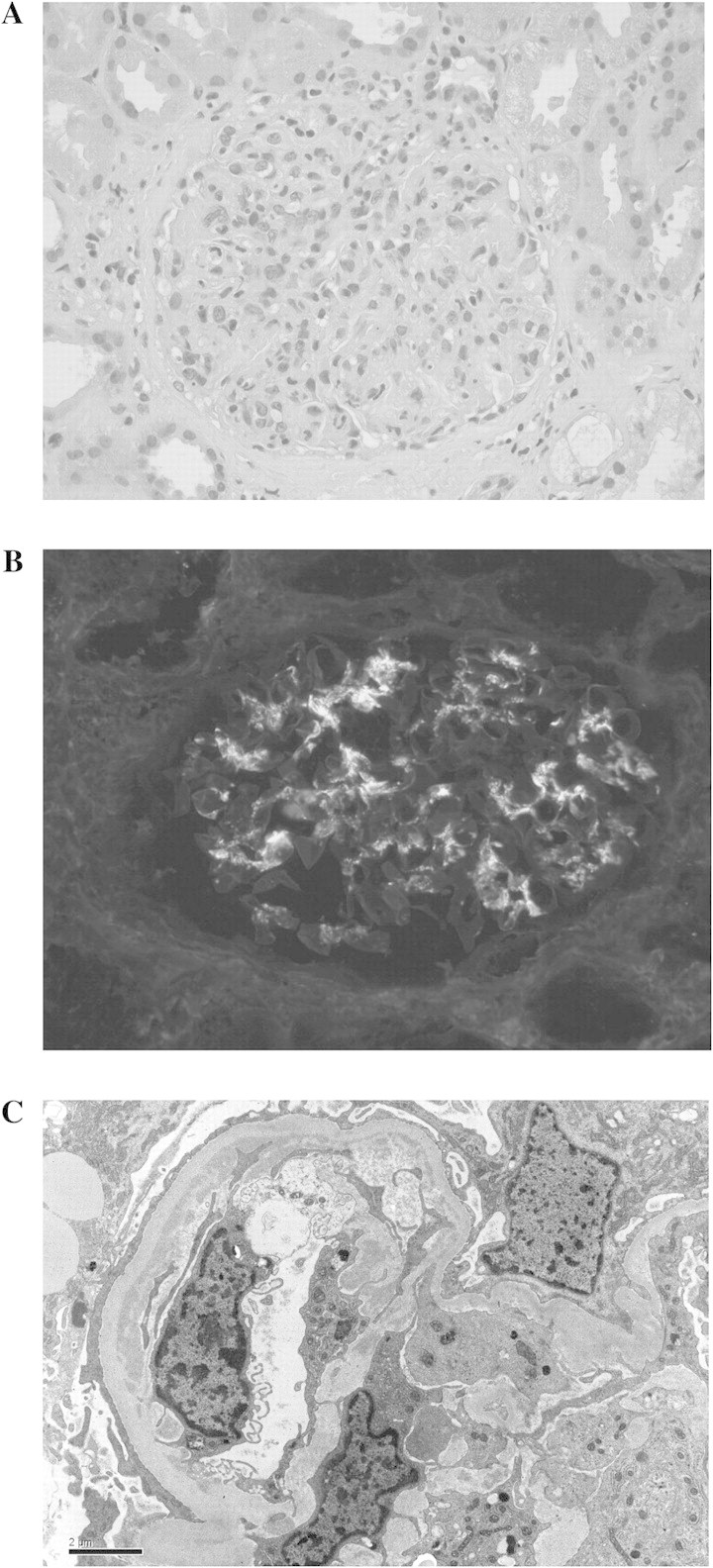
(A–C) Renal biopsy findings: (A) light microscopy with endothelial and mesangial cell proliferation with leukocyte infiltration. (B) Immunofluorescence staining for IgA in a granular pattern and mesangial deposition. (C) Electron microscopy with prominent mesangial and occasional subendothelial deposits.
Clinical follow-up
The patient was continued on IHD three times per week and i.v. vancomycin after each session of dialysis. With the finding of crescents and lack of improvement with eradication of the MSSA bacteremia, the patient was started on a trial of prednisone 60 mg daily. The patient's kidney function did not recover despite 6 weeks of treatment with i.v. vancomycin and 4 weeks of treatment with steroids. He remained on hemodialysis at his 6-month follow-up.
Case 2
Clinical history and initial laboratory data
A 69-year-old woman with a history of atrial fibrillation, hypothyroidism and chronic kidney disease secondary to contrast-induced nephropathy was transferred for treatment of a pacemaker lead infection with MSSA.
On exam, the patient was afebrile with a blood pressure of 170/80 mmHg. A 3/6 systolic murmur was noted on cardiac exam. Her skin exam was negative for rash or embolic lesion. She had 2+ pitting edema in her lower extremities. Her laboratory exam was notable for a haemoglobin of 7.05 mg/dL and a serum creatinine of 3.23 mg/dL. Urine analysis showed protein 3+ and blood 2+ by dipstick; urine microscopy showed 30 dysmorphic RBCs/high-power field, many RBCs casts and several degraded cellular casts. Oval fat bodies and numerous maltese crosses were noted. A 24-h urine collection showed 3.6 g of protein. ANA, ANCA, cryoglobulin, C3, C4, HIV and hepatitis serologies were normal. A transesophageal echocardiogram showed a mobile echodensity present on the atrial side of the right ventricular pacemaker lead. A kidney biopsy was obtained on hospital Day 3.
Kidney biopsy
Of 18 glomeruli present, 7 showed mild segmental hypercellularity, predominantly mesangial and occasionally endocapillary, with rare polymorphonuclear leukocytes. Two glomeruli were globally sclerotic, one was globally hypercellular, and the rest were unremarkable. There was moderate arteriosclerosis. Abundant eosinophilic casts without cellular reaction were present, predominantly in the medullary region. Immunohistochemistry for myoglobin A was negative. Mild tubular atrophy, interstitial fibrosis and chronic interstitial inflammation with occasional eosinophils were noted. (Figure 2A). Immunofluorescence revealed IgA, lambda and C3c deposition in the mesangium. Staining for IgG, IgM, kappa, C1q and albumin was negative (Figure 2B).
Fig. 2.
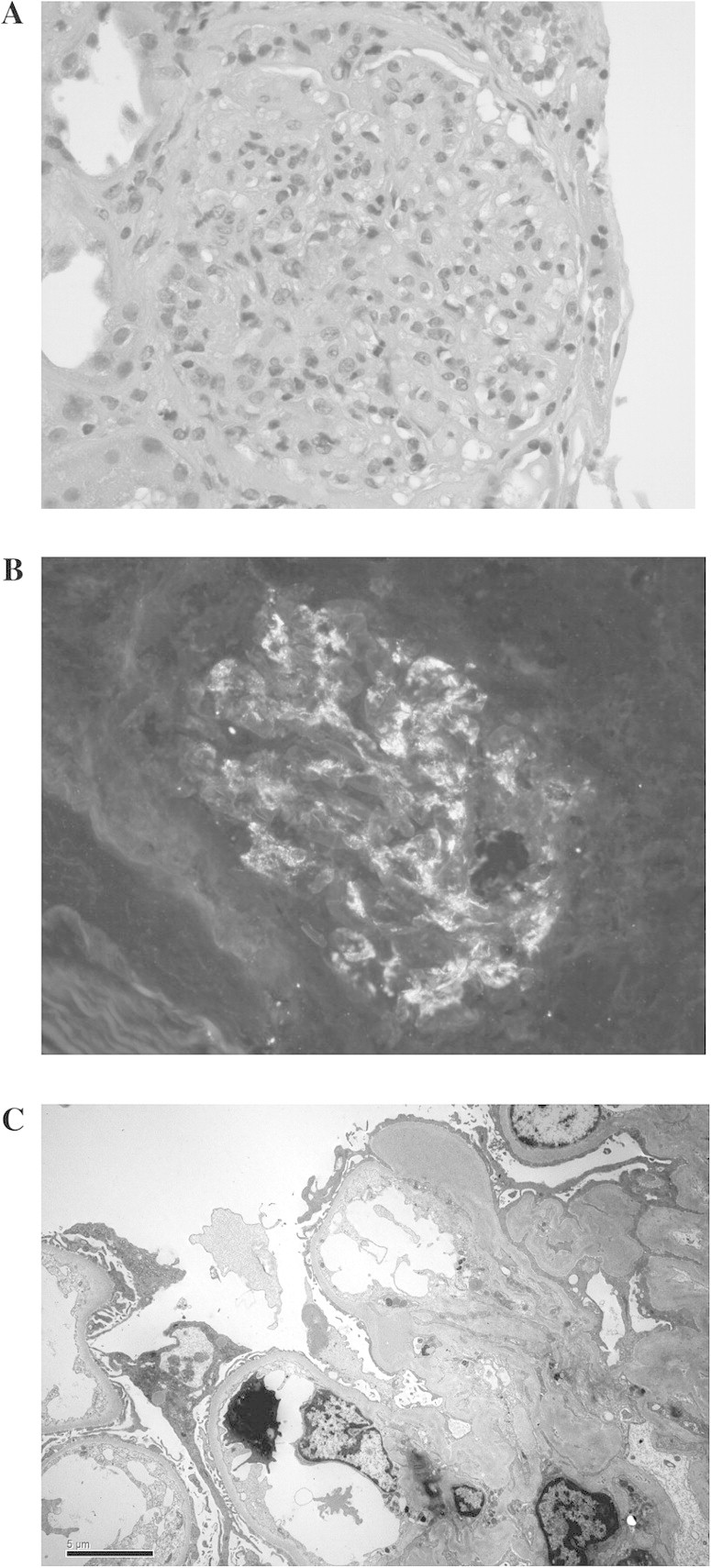
(A–C) Renal biopsy findings: (A) light microscopy with endothelial and mesangial cell proliferation with leukocyte infiltration. (B) Immunofluorescence staining for IgA in a granular pattern and mesangial deposition. (C) Electron microscopy with mesangial electron-dense deposits.
Electron microscopy showed that the glomerular basement membrane was within the normal adult range (0.36 μ). Mesangial electron-dense deposits were abundant. Subepithelial humps were not seen. There was mild epithelial foot process effacement (Figure 2C). A diagnosis of IgA-dominant focal proliferative glomerulonephritis was made.
Clinical follow-up
The patient was started on i.v. oxacillin and underwent extraction of the infected pacemaker lead. Repeat blood cultures were negative for MSSA. Her creatinine decreased to 2.2 mg/dL after 1 week from initiation of the antibiotics. She was discharged on a 6-week course of i.v. oxacillin. At 1-month follow-up, the patient’s creatinine returned to baseline of 1.5 mg/dL. Her proteinuria resolved but she continued to have microscopic hematuria.
Literature search and statistical analysis
We searched MEDLINE (December 1960–2009) using the relevant keywords such as glomerulonephritis, postinfectious glomerulonephritis, IgA nephropathy and Staphylococcus infections. Articles were reviewed to validate the postinfectious glomerulonephritis with IgA deposition. References of the included articles were searched for additional studies. After the review process, a total of 46 cases were identified (2–18) with available individual patient data. We pooled data regarding basic demographics (age, gender), comorbid conditions (hypertension, diabetes), clinical presentation (acute renal failure, hematuria, type and site of infection), laboratory values [creatinine at the time of diagnosis, urine analysis findings and complement) and clinical outcomes (death and/or need for renal replacement therapy (RRT)].
We performed descriptive statistics of all cases and expressed as mean ± SD (minimum and maximum) or median (10th and 90th percentile) for continuous variables or n (%) for categorical variables. We also explored the factors associated with the need for RRT among these cases by using the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
Discussion
Proliferative glomerulonephritis with dominant mesangial IgA deposition secondary to Staphylococcus infections is a rare clinical entity that has been described mainly in case reports. This association is rarely reported in North America but has been well documented in Japan (2). In fact, the first description dates back to 1995 when Koyoma et al. (3) described 10 such cases.
According to our review, this type of glomerulonephritis affects mainly elderly patients. The mean age at presentation was 59 ± 16, with male predominance (84%). Mean serum creatinine at the time of diagnosis was 2.53 mg/dL. A medical history of hypertension and diabetes were present in 14 and 22% of the cases, respectively. The most common sites of infection were in order of frequency: skin infection, deep-seated abscesses, surgical wounds, pneumonia and joint infection. The clinical presentation was similar to our cases with a rapidly progressive glomerulonephritis characterized by an abrupt onset of acute kidney injury, active urine sediment and heavy proteinuria. Data regarding blood pressure at the time of the glomerulonephritis diagnosis was not reported in most of the cases. Nephrotic-range proteinuria and microscopic hematuria were noted in 50 and 83% of the cases, respectively. Thirteen patients had skin lesions of purpuric nature that were histologically similar to Henoch–Schönlein purpura. Normal complement levels were noted in the majority of cases (72%) and constitute one of the most important features that distinguish this entity from other cases of APIGN. The infection was not obvious clinically at the time of onset of the glomerulonephritis in 25% of the cases and the latency period ranged between 1 to 16 weeks. Methicillin-resistant Staphylococcus aureus (MRSA) was the etiological agent in 68% of the cases. Table 1 summarizes the clinical features of these case reports.
Table 1.
Clinical characteristics of the 48 cases reported in the literature from 1980–2010a
| Number of patients | N = 48(%) |
| Age (mean) | 21–89 (59.1) |
| Sex (M/F) | 40/8 |
| Creatinine at presentation (mean) | 0.5–9.9 (2.53 mg/dL) |
| Proteinuria | |
| >3 g/day | 25 (52%) |
| <3 g/day | 19 (40%) |
| None specified | 4 (8%) |
| Hematuria | |
| Gross | 8 (17%) |
| Microscopic | 40 (83%) |
| Complement level | |
| Normal | 35 (72%) |
| Low | 12 (25%) |
| NA | 1 (3%) |
| Purpuric lesion | 13 (27%) |
| Hypertension | 7 (14%) |
| Diabetic | 11 (22%) |
| Onset after infection (week) | 1–16 (4.5) |
| Steroids use | 9 (18%) |
| Type of infection | |
| MRSA | 33 (68%) |
| MSSA | 9 (18%) |
| Other | 6 (14%) |
| Outcome | |
| RRT | 16 |
| Death | 7 |
NA, non available.
The pathogenesis of this rare disease is not entirely clear. Proposed mechanisms include the role of Staphylococcus superantigens (3). Staphylococcus enterotoxin may act as a superantigen by binding directly to the major histocompatibility complex Class II molecules on the membrane of antigen-presenting cells and on the specific Vβ chain of T-cell receptors (19). This process leads to proliferation and massive activation of T cells with release of lymphokines including interleukin 1, 2 and 6, tumor necrosis factor and interferon-γ (20). This burst of cytokines activates B cells that will produce polyclonal IgA and IgG, which will result in the formation of immune complex and deposition in the glomeruli (21).
The histological features on light microscopy were of various types of mesangial and/or endocapillary proliferation. Fibrocellular crescents were reported in 35% of the cases. Interstitial fibrosis was commonly found in 70% of the patients with a mean grade of 1+. Hyaline thrombi that resemble cryoglobulin were noted in two cases (1). All cases with diabetes had features of diabetic glomeruloscelrosis. Immunofluorescence is classically noted for a 2+ IgA predominant or codominant with IgG immune complex deposition in the mesangium in a granular pattern. Sixty-one percent of the cases had a 2+ intensity for the IgA staining and 32% of the cases had a 1+ IgG staining. Kappa and lambda were 2+ stained in 45 and 68% of the cases, respectively. The stronger intensity of staining for IgA and lambda might favor the diagnosis of IgA nephropathy over postinfectious glomerulonephritis when cases of post-Staphylococcus IgA-dominant glomerulonephritis were compared to control with primary IgA nephropathy (1). On electron microscopy, various numbers of large mesangial electron-dense deposits were seen in most of the cases. Large subepithelial ‘humps’ and subendothelial deposits classically described with other APIGN were unusual.
The morphological features described above are clearly more similar to those found with idiopathic IgA nephropathy than to those found with another form of postinfectious glomerulonephritis such as poststreptococal glomerulonephritis (1). It is possible that cases have been misdiagnosed with IgA nephropathy or non-diagnosed for example in a diabetic patient in whom an infected ulcer was overlooked. Age at onset, type and latency of infection, renal function and amount of proteinuria are important clinical clues to distinguish primary IgA nephropathy from Staphylococcus infection-associated glomerulonephritis. On subgroup analysis (data not shown), there was no difference between the MRSA and the MSSA cases in term of clinical presentation and outcomes. It is unclear why MRSA was more prevalent. One possible explanation could be related to susceptibility of MSSA to routine antibiotics use, which leads to rapid eradication of the infection and subsequent prevention of the development of the glomerulonephritis.
The outcome of a patient with IgA-dominant post-Staphylococcus infection is variable. In our review, all patients were treated with anti-staphyloccous antibiotics. Renal recovery was noted in 27 (56%) patients. RRT and death were noted in 16 (33%) and 7 (14%) patients, respectively. Nine patients additionally received steroids (18%). Of the nine patients who received steroids, two patients died, another patient required RRT and the six others had improvement in their kidney function. The role of steroids in treating this rare glomerulonephritis remains unclear at this point. Satoskar et al. (2) have suggested that steroids should be avoided because of the increased risk of worsening of the infections. Although occasional case reports advocate the use of steroids (15), further randomized studies are needed to better define the role of steroids. However, such studies are difficult to perform with the small number of cases reported.
Predictors of long-term outcome after postinfectious glomerulonephritis are not well defined (22). Understanding the limitation of collecting and combining data from published case reports, we explored the factors associated with the need for RRT. This need was significantly associated with the presence of pre-existing diabetes, hypertension and fibrosis on kidney biopsy (Table 2). Even though not statistically significant, higher serum creatinine at presentation and low complements might anticipate the need for dialysis in these patients.
Table 2.
Predictors of RRT based on the 48 cases reported in the literaturea
| RRT N = 16 (%) | No RRT N = 32 (%) | P-value | |
| Age | 66.0 | 61.5 | 0.35b |
| Female | 2 (13) | 6 (19) | 0.70 |
| Hypertension | 5 (31) | 2 (6) | 0.033 |
| Diabetes | 8 (50) | 3 (9) | 0.003 |
| Creatinine at presentation | 3.8 | 1.6 | 0.064b |
| Hematuria (microscopic) | 13 (81) | 27 (84) | 0.99 |
| Proteinuria | 0.66 | ||
| >3 g/day | 7 (44) | 18 (56) | |
| <3 g/day | 7 (44) | 12 (38) | |
| NA | 2 (13) | 2 (6) | |
| Low complement | 7 (44) | 5 (16) | 0.075 |
| Latent period weeks | 2.0 | 4.0 | 0.43b |
| Skin lesion | 2 (13) | 11 (34) | 0.17 |
| Crescent | 3 (20) | 11 (38) | 0.31 |
| Fibrosis | <0.001 | ||
| Absent | 0 (0) | 14 (48) | |
| 1+ | 5 (33) | 13 (45) | |
| 2+ | 7 (47) | 2 (7) | |
| 3+ | 3 (20) | 0 (0) |
NA, non available.
Wilcoxon rank-sum test.
Conclusions
IgA-dominant Staphylococcus glomerulonephritis is a rare clinical entity with unique clinical and morphologic features that all nephrologists need to be aware of for making the correct diagnosis. The occurrence of a rapidly progressive glomerulonephritis in an elderly patient with mesangial deposit of IgA and normal complement should raise suspicion for a Staphylococcus infection-associated glomerulonephritis. The presence of diabetes, hypertension and interstitial fibrosis were associated with worse outcomes. Further studies are needed to determine why only a subset of patients with Staphylococcus infection developed such rare glomerulonephritis.
Acknowledgments
S.D.N. is supported by the National Institutes of Health, the National Center for Research Resources, Multidisciplinary Clinical Research Career Development program Grant #: RR024990.
Conflict of interest statement. None declared.
References
- 1.Nasr SH, Markowitz GS, Stokes MB, et al. Acute postinfectious glomerulonephritis in the modern era: experience with 86 adults and review of the literature. Medicine (Baltimore) 2008;87:21–32. doi: 10.1097/md.0b013e318161b0fc. [DOI] [PubMed] [Google Scholar]
- 2.Satoskar AA, Nadasdy G, Plaza JA, et al. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol. 2006;1:1179–1186. doi: 10.2215/CJN.01030306. [DOI] [PubMed] [Google Scholar]
- 3.Koyama A, Kobayashi M, Yamaguchi N, et al. Glomerulonephritis associated with MRSA infection: A possible role of bacterial superantigen. Kidney Int. 1995;47:207–216. doi: 10.1038/ki.1995.25. [DOI] [PubMed] [Google Scholar]
- 4.Long JA, Cook WJ. IgA deposits and acute glomerulonephritis in a patient with staphylococcal infection. Am J Kidney Dis. 2006;48:851–855. doi: 10.1053/j.ajkd.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Riley AM, Wall BM, Cooke CR. Favorable outcome after aggressive treatment of infection in a diabetic patient with MRSA-related IgA nephropathy. Am J Med Sci. 2009;337:221–223. doi: 10.1097/MAJ.0b013e318184a4a1. [DOI] [PubMed] [Google Scholar]
- 6.Nasr SH, Share DS, Vargas MT, et al. Acute post staphylococcal glomerulonephritis superimposed on diabetic glomerulosclerosis. Kidney Int. 2007;71:1317–1321. doi: 10.1038/sj.ki.5002135. [DOI] [PubMed] [Google Scholar]
- 7.Nasr SH, Markowitz GS, Whelan JD, et al. IgA-dominant acute poststaphylococcal glomerulonephritis complicating diabetic nephropathy. Hum Pathol. 2003;34:1235–1241. doi: 10.1016/s0046-8177(03)00424-6. [DOI] [PubMed] [Google Scholar]
- 8.Nagaba Y, Hiki Y, Aoyama T, et al. Effective antibiotic treatment of methicillin-resistant Staphylococcus aureus-associated glomerulonephritis. Nephron. 2002;92:297–303. doi: 10.1159/000063309. [DOI] [PubMed] [Google Scholar]
- 9.Denton MD, Digumarthy SR, Chua S, et al. Case records of the Massachusetts General Hospital. Case 20-2006. An 84-year-old man with staphylococcal bacteremia and renal failure. N Engl J Med. 2006;354:2803–2813. doi: 10.1056/NEJMcpc069012. [DOI] [PubMed] [Google Scholar]
- 10.Griffin MD, Björnsson J, Erickson SB. Diffuse proliferative glomerulonephritis and acute renal failure associated with acute staphylococcal osteomyelitis. J Am Soc Nephrol. 1997;8:1633–1639. doi: 10.1681/ASN.V8101633. [DOI] [PubMed] [Google Scholar]
- 11.Handa T, Ono T, Watanabe H, et al. Glomerulonephritis induced by methicillin-sensitive Staphylococcus aureus infection. Clin Exp Nephrol. 2003;7:247–249. doi: 10.1007/s10157-003-0240-4. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Nogaki F, Oida E, et al. Glomerulonephritis induced by methicillin-resistant Staphylococcus aureus infection that progressed during puerperal period. Clin Exp Nephrol. 2007;11:92–96. doi: 10.1007/s10157-006-0444-5. [DOI] [PubMed] [Google Scholar]
- 13.Yoh K, Kobayashi M, Hirayama A, et al. A case of superantigen-related glomerulonephritis after methicillin-resistant Staphylococcus aureus (MRSA) infection. Clin Nephrol. 1997;48:311–316. [PubMed] [Google Scholar]
- 14.Kai H, Shimizu Y, Hagiwara M, et al. Post-MRSA infection glomerulonephritis with marked Staphylococcus aureus cell envelope antigen deposition in glomeruli. J Nephrol. 2006;19:215–219. [PubMed] [Google Scholar]
- 15.Okuyama S, Wakui H, Maki N, et al. Successful treatment of post-MRSA infection glomerulonephritis with steroid therapy. Clin Nephrol. 2008;70:344–347. doi: 10.5414/cnp70344. [DOI] [PubMed] [Google Scholar]
- 16.Pola E, Logroscino G, De Santis V, et al. Onset of Berger disease after Staphylococcus aureus infection: septic arthritis after anterior cruciate ligament reconstruction. Arthroscopy. 2003;19:E29. doi: 10.1053/jars.2003.50118. [DOI] [PubMed] [Google Scholar]
- 17.Spector DA, Millan J, Zauber N, et al. Glomerulonephritis and Staphylococcal aureus infections. Clin Nephrol. 1980;14:256–261. [PubMed] [Google Scholar]
- 18.Zeledon JI, McKelvey RL, Servilla KS, et al. Glomerulonephritis causing acute renal failure during the course of bacterial infections. Histological varieties, potential pathogenetic pathways and treatment. Int Urol Nephrol. 2008;40:461–470. doi: 10.1007/s11255-007-9323-6. [DOI] [PubMed] [Google Scholar]
- 19.Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int. 2004;66:121–132. doi: 10.1111/j.1523-1755.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoh K, Kobayashi M, Yamaguchi N, et al. Cytokines and T-cell responses in superantigen-related glomerulonephritis following methicillin-resistant Staphylococcus aureus infection. Nephrol Dial Transplant. 2000;15:1170–1174. doi: 10.1093/ndt/15.8.1170. [DOI] [PubMed] [Google Scholar]
- 21.Gjörloff A, Fischer H, Hedlund G, et al. Induction of interleukin-1 in human monocytes by the superantigen staphylococcal enterotoxin A requires the participation of T cells. Cell Immunol. 1991;137:61–71. doi: 10.1016/0008-8749(91)90056-h. [DOI] [PubMed] [Google Scholar]
- 22.Moroni G, Pozzi C, Quaglini S, et al. Long-term prognosis of diffuse proliferative glomerulonephritis associated with infection in adults. Nephrol Dial Transplant. 2002;17:1204–1211. doi: 10.1093/ndt/17.7.1204. [DOI] [PubMed] [Google Scholar]


