Abstract
Cytomegalovirus (CMV) is an important and well-described opportunistic virus in renal transplant recipients (RTRs) with infection occurring mainly after the first month post-renal transplant. CMV can present as primary infection, reinfection or reactivation of latent disease. Skin manifestations are rare and variable, and diagnosis is often delayed. We present one case of skin CMV ulcer of perineal areas without systemic symptoms of CMV disease and a negative quantitative polymerase chain reaction. This case serves to illustrate the protean nature of CMV disease in RTR.
Keywords: cutaneous, cytomegalovirus, renal transplant
Background
Cytomegalovirus (CMV) disease represents an important cause of morbidity and mortality in renal transplant recipients (RTRs). CMV disease can present as primary infection, reinfection or reactivation of latent disease. Patients at highest risk of symptomatic disease are CMV-seronegative recipients matched with CMV-seropositive donor and patients treated with anti-lymphocyte agents. Classically, CMV infection presents with fever, constitutional symptoms, visceral disease and laboratory evidence of bone marrow suppression. Diagnosis of CMV infection is based on the demonstration of the virus in the blood and the quantification of viral load [1]. Detection of viral genetic material using polymerase chain reaction (PCR) is an excellent diagnostic test for CMV infection with a sensitivity in serum or plasma of 50–100% and specificity of 45–65%.
Case report
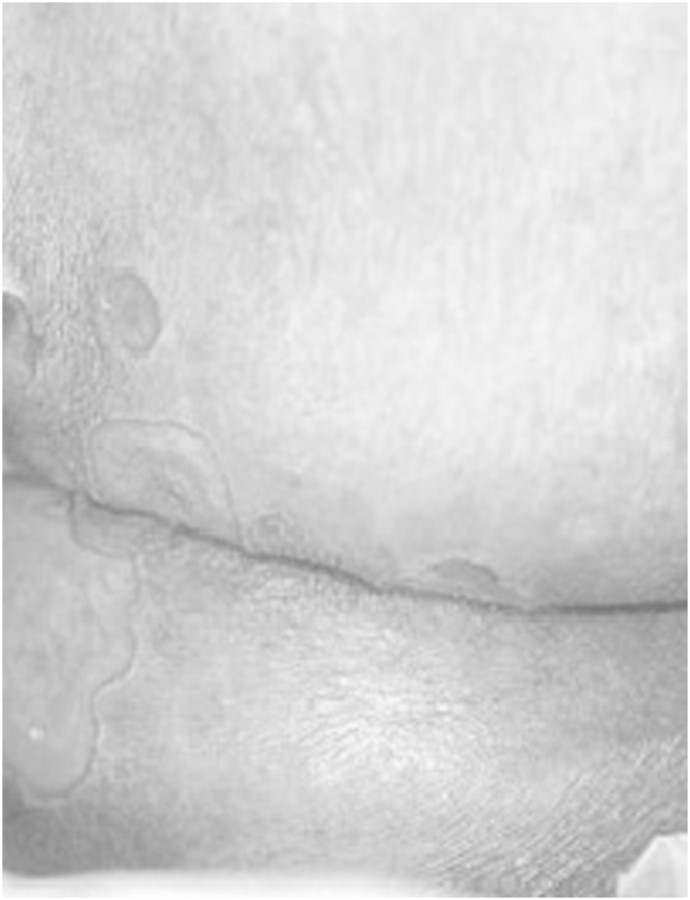
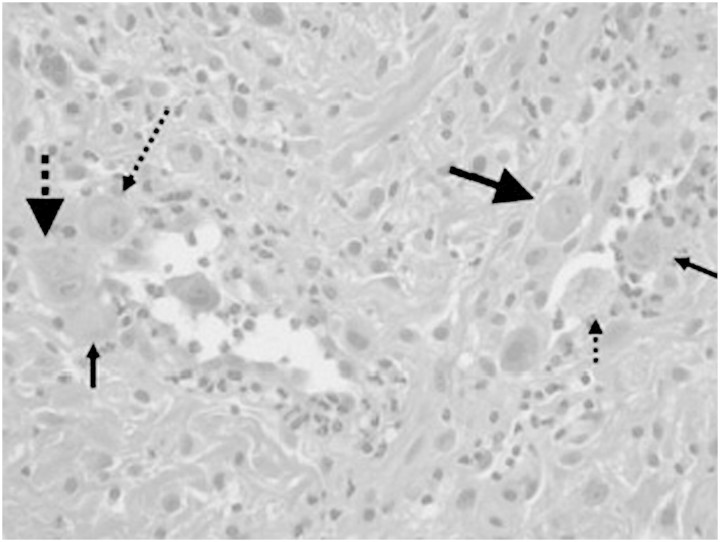
A 54-year-old woman presented 1 month post-renal transplant with a several-day history of painful ulceration of the median aspect of her gluteus (Figure 1). She had received a renal transplant in January 2010 for end-stage renal failure secondary to polycystic kidney disease. Both the donor and recipient were CMV-seropositive. She was on treatment with immunosuppressive therapy based on cyclosporine (CyA) (dose was adjusted to maintain trough blood levels between 100 and 200 mg/mL), steroid (methylprednisolone, dose was 16 mg/day) and mycophenolate mofetil (MMF) (dose was 2 g/day). She received induction therapy with basiliximab before surgery. On examination, she was apyretic, and there were no other significant findings, apart from the lesions involving her gluteus: an exophytic ulcer surrounded by verrucous borders along the right border of the intergluteus and eroded papules around it, and a furrow with two smaller lesions on the upper side. Haematological results indicated a red blood cell count (RBC) of 3.8 × 1012/mm3, haematocrit of 35.5%, haemoglobin of 12.1 g/dL, white blood cell count (WBC) of 7.23 × 109/mm3 and thrombocytes of 198 000 × 109/mm3. The leucocyte formula was as follows: neutrophils 73.9%, basophils 0.7%, eosinophils 0.1%, lymphocytes 14.5%, monocytes 10.8%, serum creatinine 135 μmol/L, blood urea nitrogen 0.75 g/L and 24-h creatinine clearance 60 mL/min. Erythrocyte sedimentation rate was 22. Urinalysis showed absence of proteinuria or haematuria and revealed only hyaline casts. PCR CMV was negative. A presumptive diagnosis of bacterial infection was made, but the ulcers were refractory to conventional measures such as cleansing and the use of ointments. After 2 weeks, PCR CMV was negative, yet other haematological and urinary results were stable. A presumptive diagnosis of virus infection was made. An ulcer swab was positive for CMV (PCR = 16 500 copies/mL) and for HSV2 (PCR = 11 000 copies/mL). A prepared section of paraffin-embedded tissues from the formalin-fixed biopsy was stained with haematoxylin and eosin and showed inflammatory granulated tissue with extravasation of erythrocytes and some neutrophils. Some cells were characteristically enlarged with large purplish intranuclear inclusions surrounded by clear halo and occasional eosinophilic cytoplasmic inclusions, suggestive of CMV infection (Figure 2). One week later, PCR CMV rapidly increased (271 000 copies/mL and 400 000 copies/mL 2 weeks later), and the WBC decreased (3950 × 109/mm3). The leucocyte formula and other parameters were stable. Intravenous ganciclovir therapy was administered (dose was 2.5 mg/kg daily) for 2 weeks, and MMF was stopped. PCR CMV rapidly decreased (15 000 copies/mL), and WBC increased (5150 × 109/mm3), with a complete recovery of the lesions within 3 months. After discontinuation of intravenous ganciclovir, the patient was treated with oral valganciclovir therapy, 900 mg daily for another 3 months.
Fig. 1.
Exophytic ulcer surrounded by verrucous borders along the right border of intergluteus and eroded papules around it, and a furrow with some smaller lesions on the upper side.
Fig. 2.
Some endothelial cells were characteristically enlarged with large purplish intranuclear inclusions surrounded by clear halo and occasional eosinophilic cytoplasmic inclusions, suggestive of CMV infection.
Discussion
Skin disease remains a rare manifestation of reactivated CMV disease in any setting [2]. It usually presents as generalized maculopapular eruptions, but ulcers, nodules, vesicles, petechiae and plaques may also be seen and can mimic other skin eruptions and cutaneous viral infections, especially herpetic infections. Ulceration, particularly involving the genital, perineum and perianal areas [3], as well as necrosis of the mucosal membranes can occur in more severe cases. However, none of these appearances is pathognomonic for CMV [2,4]. It has been postulated that the dermis is relatively inhospitable to CMV with skin disease only occurring in the significantly immunocompromised host [2]. As such, skin infection has generally been associated with disseminated disease, thus reflecting the overall degree of immunosuppression in the patients, although localized disease can occur and may be associated with a better outcome [4]. The predominant defence against CMV is the MHC-restricted cytotoxic T cells, with growing evidence for the significant contributory role played by CD4 + T cells and humoral immunity [5]. Immunosuppressive therapy suppresses cytotoxic cells in a dose-dependent effect and thus enhances viral replication once stimulated by other factors. There are two possible explanations for such CMV skin ulcers: the virus resides in the gastrointestinal tract in a latent stage and then it infects the perineum skin via faecal shedding when it is reactivated [6], or there is a reactivation of a local latent virus in endothelial cells during endothelial colonization on the path to haematogenous dissemination. Accumulating evidence suggests that CMV may target a long-lived cell population, such as CD34 + haematopoietic cells, which gives rise to monocyte-derived macrophages and dendritic cells as a site of latency [7,8]. Furthermore, TNF-α has been suggested to play an important role in CMV reactivation [7,8]. In fact, a strong correlation between an elevated TNF-α plasma level and CMV antigenaemia has been reported in RTRs [9] in wound healing in a TNF-α-rich environment, and it is present in the early stages of wound bed repair [10]. As wound healing progresses, granulation tissues with many macrophage infiltrates and hypervascularity develop. During this process, CD34 + bone marrow progenitors differentiate macrophages in the lesion. Moreover, endothelial progenitor cells derived from bone marrow have been isolated from circulating mononuclear cells and were shown to be incorporated into the foci of neovascularization. Taken together, the above observations suggest that it is likely that latent CMV is reactivated in the ulcer on the path to haematogenous dissemination. Accordingly, infiltrated CD34 + bone marrow cells with latent CMV infection differentiate macrophages and some endothelial cells in the ulcer. Then, in the TNF-α-rich environment, the latent CMV is reactivated in the wound bed, and then, CMV antigenaemia develops. Finally, CMV dissemination occurs and may cause severe disease. Histological examination of tissue specimens remains the gold standard diagnostic test for CMV tissue-invasive disease. However, pathological examination of CMV infection is a slow process. Skin manifestation responded well to appropriate antiviral therapy and reduction in net immunosuppression. There are no published data specifically addressing the type and duration of antiviral treatment of skin CMV disease in the immunocompromised hosts. In general, uncontrolled studies suggest that 2–3 weeks of ganciclovir is adequate for treatment of organ involvement in CMV disease. The presence of a CMV skin ulcer can represent the first sign of systemic CMV infection. This case serves to illustrate the protean nature of CMV disease in RTR, and we must remain vigilant to the possibility of an atypical presentation of CMV and initiate treatment as soon as possible.
Acknowledgments
The authors thank Anna Morra for her technical assistance.
Conflict of interest statement. None declared.
References
- 1.Tong CY, CueVas LE, Williams H. Prediction and diagnosis of cytomegalovirus disease in renal transplant recipients using qualitative polymerase chain reaction. Transplantation. 2000;69:985–991. doi: 10.1097/00007890-200003150-00054. [DOI] [PubMed] [Google Scholar]
- 2.Trimarchi H, Casas G, Jordan R, et al. Cytomegalovirus maculopapular eruption in a kidney transplant patient. Transpl Infect Dis. 2001;3:47–50. doi: 10.1034/j.1399-3062.2001.003001047.x. [DOI] [PubMed] [Google Scholar]
- 3.Drago F, Aragone MG, Lugani C, et al. Cytomegalovirus infection in normal and immunocompromised humans. Dermatology. 2000;200:189–195. doi: 10.1159/000018381. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY. Cytomegalovirus infection involving the skin in immunocompromised hosts. A clinicopathologic study. Am J Clin Pathol. 1989;92:961–700. doi: 10.1093/ajcp/92.1.96. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 6.Horn T, Hood A. Clinically occult cytomegalovirus present in skin biopsy specimens in immunosuppressed hosts. J Am Acad Dermatol. 1989;21:781–784. doi: 10.1016/s0190-9622(89)70254-1. [DOI] [PubMed] [Google Scholar]
- 7.Kondo K, Xu J, Mocarki ES. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fietze E, Prosch S, Reinke P, et al. Cytomegalovirus infection in transplant recipients. Transplantation. 1994;58:675–680. [PubMed] [Google Scholar]
- 10.Freedberg IM, Tomic-Canic M, Komine M, et al. Keratins and keratinocyte activation cycle. J Invest Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]