Abstract
Oligomeganephronia is classified as a subgroup of renal hypoplasia, characterized by histopathologic abnormalities which progress to end-stage renal disease (ESRD) by school age. We describe three adult cases of oligomeganephronia who have not yet developed ESRD. We performed a renal biopsy in all of them. The pathological features, consisting of a reduced number of enlarged glomeruli, were diagnostic of oligomeganephronia. It was assumed that the condition had not progressed to ESRD in the patients because the degree of loss of glomeruli may have been milder than that in typical cases of oligomeganephronia.
Keywords: late-onset, low birth weight, oligomeganephronia, renal hypoplasia
Introduction
Oligomeganephronia is a type of renal hypoplasia characterized by a reduced number of nephrons and hypertrophic glomeruli enlarged in diameter [1]. In regard to the aetiology, while it is not yet clearly known, inadequate embryonic development of the metanephric blastema during the 14–20-week period interferes with the formation of the nephron. Placental shunts and intravascular coagulation were found in some cases [2]. Low birth weight (LBW) and intrauterine growth retardation (IUGR) are also related to oligomeganephronia [3]. Cases related to 4p monosomy [4], PAX-2 gene mutations [5] and hepatocyte nuclear factor-1 (HNF-1) mutation carriers [6] have also been reported. In most cases, it begins with polydipsia and polyuria in early childhood, and then leads to end-stage renal disease (ESRD) by school age (early-onset type). In some cases, asymptomatic proteinuria prompts closer examination, including kidney biopsy, and leads to the diagnosis of oligomeganephronia by school age (late-onset type). We report, for the first time, three adult oligomeganephronia patients who have not yet developed ESRD. We performed renal biopsy in all of them and calculated the glomerular number by using the Lumina Vision system (Mitani Corporation, Fukui, Japan).
Case reports
Case 1
A 36-year-old male, with no symptoms and no past medical history, exhibited hypertension (166/113 mmHg) during a medical check-up. His primary doctor started him, in a phased manner, on amlodipine 5 mg/day, candesartan 2 mg/day and carvedilol 2.5 mg/day. He was subsequently referred to our centre. The physical findings on admission were almost normal (blood pressure 138/93 mmHg); however, his serum creatinine level was 2.65 mg/dL. Urinalysis showed proteinuria (1 +) but no haematuria. On ultrasonography, both kidneys were atrophic and showed a diffuse increase of echogenicity. The number (1.29/mm2) and diameter (200 μm) of the glomeruli were diagnostic of oligomeganephronia. Continuing with the safe treatment, he has maintained his serum creatinine level at 2.5–3.0 mg/dL for 3.5 years.
Case 2
In a 19-year-old female, proteinuria (+) and occult blood in the urine (±) were detected for the first time at a school urinalysis screening. After initiating temocapril 1 mg/day, no improvement of proteinuria was seen, and she was referred to our centre. The physical findings on admission were almost normal. Her serum creatinine level was 1.14 mg/dL. Urinalysis revealed proteinuria (2 +) but no haematuria. On ultrasonography, both kidneys were atrophic, with a diffuse increase of echogenicity. The number (0.76/mm2) and diameter (310 μm) of the glomeruli were diagnostic of oligomeganephronia. One of the glomeruli showed spherical sclerosis; however, none showed crescent formation or adhesions. Segmental sclerosis surrounding the vascular pole and enlargement of the juxtaglomerular apparatus were found. Mild tubular atrophy and interstitial fibrosis were noted (9.36%). After the diagnosis, losartan 12.5 mg/day and dilazep 300 mg/day were added to temocapril. The serum creatinine was maintained at 1.1–1.3 mg/dL for 3 years.
Case 3
In a 21-year-old male, proteinuria was detected for the first time during a school urinalysis screening when he was 15 years old. Suspected of having familial renal disease, he was admitted to our centre. The physical findings on admission were almost normal. His serum creatinine level was 1.20 mg/dL. Urinalysis revealed proteinuria (3 +) but no haematuria. The number (0.97/mm2) and diameter (270 μm) of the glomeruli were diagnostic of oligomeganephronia. After the diagnosis, we initiated the patient on losartan 12.5 mg/day. The serum creatinine was maintained at 1.1–1.2 mg/dL for 1.5 years.
Discussion
LBW is defined by the World Health Organization as a birth weight of < 2500 g. LBW has especially attracted attention as occurring more frequently in disadvantaged communities, in which there is often a disproportionately high incidence of adult cardiovascular disease, hypertension, diabetes mellitus and kidney disease [7]. It has been hypothesized that poor fetal growth results in a reduced number of nephrons and an increased incidence of renal disease later in life. Fewer nephrons with subsequent hyperfiltration and glomerulosclerosis in the remaining nephrons could lead to accelerated ageing and early loss of renal function. This hypothesis is supported by the results of human post-mortem and animal studies, where low birth weight is found to be correlated with fewer nephrons, and thereby reduced kidney weight/volume [8]. Case 2 was a LBW baby who was raised in an incubator because of weak suckling. Additionally, focal segmental glomerulosclerosis (FSGS, perihilar type) was found in the specimen from Case 2. Hodgin et al. [9] also reported an association between very low birth weight (VLBW) and FSGS. In this report, we analysed the number of glomeruli per renal cortical area [normal value (mean ± SD): 3.2 ± 1.2/mm2] [10] and glomerular diameter [normal value (mean ± SD): 201 ± 28 μm]. In two of our cases (Case 2 and Case 3), the results of a school urinalysis screening led to further detailed investigations (Table 1). However, Case 1 was unusual because hypertension led to further scrutiny. The blood pressure abnormality did not develop until 35 years of age, and the patient remained free of symptoms, presumably because the reduction of glomerular number was mild. This was probably due to the fact that the enlargement of the glomerular diameter was also mild in comparison with typical cases. Use of renin–angiotensin system inhibitors might have kept the serum creatinine values from worsening in these patients. In conclusion, histopathological and morphometric studies of kidney biopsies, conducted based on abnormal results of school urinalysis screening and detection of hypertension during medical check-ups, revealed a reduced number of enlarged glomeruli (Figure 1). These cases showed that the spectrum of oligomeganephronia may be wider than previously noted. The condition was associated with LBW in one of our cases.
Table 1.
Clinical and pathological findings in late-onset oligomeganephronia
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age/sex | 36/male | 19/female | 21/male |
| Past history | Taken care in NICU | ||
| Family history | Father, ESRD; sister, MPGN | ||
| Gestation (weeks)/birth weight (g) | 37/2700 (median) | 41/2410 (light for date) | 41/3405 (median) |
| Body mass index (kg/m2) | 20.9 | 21.8 | 19.1 |
| Initiation | Hypertension at medical check-up | Proteinuria at school urinalysis | Proteinuria at school urinalysis |
| Initial→recent blood pressure (mmHg) | 166/113→110/70 | 98/60→96/60 | 132/70→110/70 |
| Urinalysis | No proteinuria or haematuria | Proteinuria (1 +), RBC 1/1–4 HPF | Proteinuria (3 +), no haematuria |
| 24-h urine protein (g)/urine volume (mL/day) | 0.18/1800 | 0.53/1090 | 0.65/1810 |
| Initial/recent serum creatinine (mg/dL) | 2.65/2.89 | 1.14/1.20 | 1.20/1.12 |
| Echo findings (right; left mm) | Atrophic (89 × 41; 93 × 47), high echogenicity | Atrophic (87 × 29; 80 × 47), high echogenicity | Normal (106 × 45; 105 × 42) |
| Medication | ACEI, ARB, CCB, αβ-blocker, aspirin | ACEI, ARB, dilazep | ARB |
| Follow-up at our centre (years) | 3.5 | 3 | 1.5 |
| Pathologic findings | |||
| Glomerular numbera (/mm2) | 1.29 | 0.76 | 0.97 |
| Maximum of glomerular diameterb (μm) | 200 | 310 | 270 |
| Number of glomerular sclerosis (/total) | 2/10 | 1/4 | 0/6 |
| Focal segmental glomerulosclerosis | (perihilar type) | ||
| Tubular atrophy and fibrosis (% of cortex) | 2.96 | 9.36 | 10.1 |
| Fluorescence staining | Non-specific IgM deposition | Non-specific IgM deposition | Non-specific IgM deposition |
| Electron microscopic examination | Partial foot process fusion | Partial foot process fusion | Almost normal |
NICU, neonatal intensive care unit; ESRD, end-stage renal disease.
Normal glomerular number (mean ± SD): 3.2 ± 1.2/mm2.
Normal glomerular diameter (mean ± SD): 201 ± 28 μm.
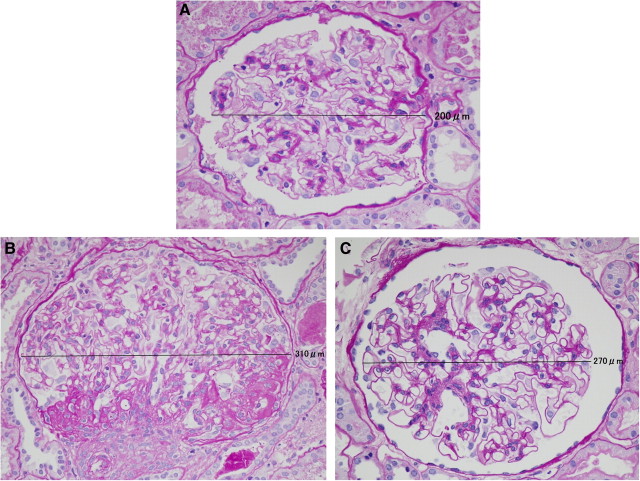
Fig. 1.
Renal biopsy showed a low number of enlarged glomeruli indicating oligomeganephronia. Light microscopy of biopsy specimens stained with periodic acid–Schiff (× 400). (A) Case 1. (B) Case 2. (C) Case 3.
Conflict of interest statement. None declared.
References
- 1.Royer P, Habib R, Courtecuisse V, et al. Bilateral renal hypoplasia with oligonephronia (study of 21 cases) Arch Fr Pédiatr. 1967;24:249–268. [PubMed] [Google Scholar]
- 2.Weir MR, Salinas JA, Rawlings PC. Intrauterine twin demise and oligomeganephronia. Nephron. 1985;40:482–484. doi: 10.1159/000183525. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? Am J Kidney Dis. 1995;26:91–98. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 4.Park SH, Chi JG. Oligomeganephronia associated with 4p deletion type chromosomal anomaly. Pediatr Pathol. 1993;13:731–740. doi: 10.3109/15513819309048260. [DOI] [PubMed] [Google Scholar]
- 5.Salomon R, Tellier AL, Attie-Bitach T, et al. PAX2 mutations in oligomeganephronia. Kidney Int. 2001;59:457–462. doi: 10.1046/j.1523-1755.2001.059002457.x. [DOI] [PubMed] [Google Scholar]
- 6.Sagan JV, Bostad L, Njolstad PR, et al. Enlarged nephrons and severe nondiabetic nephropathy in hepatocyte nuclear factor-1beta (HNF-1beta) mutation carriers. Kidney Int. 2003;64:793–800. doi: 10.1046/j.1523-1755.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 7.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;97:68–77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 8.Fuller KE. Low birth-weight infants: the continuing ethnic disparity and the interaction of biology and environment. Ethn Dis. 2000;10:432–445. [PubMed] [Google Scholar]
- 9.Hodgin JB, Rasoulpour M, Markowitz GS, et al. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:71–76. doi: 10.2215/CJN.01700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuboi N, Kawamura T, Koike K, et al. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5:39–44. doi: 10.2215/CJN.04680709. [DOI] [PMC free article] [PubMed] [Google Scholar]