Abstract
We report a case of a heretofore healthy 18-year-old man who presented with a 2-day history of nausea, vomiting and stomach ache while taking creatine monohydrate for bodybuilding purposes. The patient had acute renal failure, and a renal biopsy was performed to determine the cause of increased creatinine and proteinuria. The biopsy showed acute tubular necrosis. In the literature, creatine monohydrate supplementation and acute tubular necrosis coexistence had not been reported previously. Twenty-five days after stopping the creatine supplements, the patient recovered fully. Even recommended doses of creatine monohydrate supplementation may cause kidney damage; therefore, anybody using this supplement should be warned about this possible side effect, and their renal functions should be monitored regularly.
Keywords: acute renal failure, creatine monohydrate, renal biopsy
Background
Creatine supplements are used as a possible performance-enhancing substance by athletes, bodybuilders, and others who wish to gain muscle mass. Most of the studies that have examined the potential toxicity of creatine supplements have not found evidence of any side effects when consumed at recommended doses [1–4]. In the literature, creatine monohydrate supplementation and acute tubular necrosis (ATN) coexistence has not been reported previously. Here, we report a patient who had acute renal failure while taking recommended doses of creatine supplements.
Case report
A heretofore healthy 18-year-old man presented with a 2-day history of nausea, vomiting and stomach ache while consuming induction (20 g/day for 5 days) and maintenance (1 g/day for the next 6 weeks) dosages of creatine monohydrate for bodybuilding purposes. He did not have any significant past medical or family history. At physical examination, he weighed 74 kg, and his BMI was 24.18 kg/m2. His blood pressure was 150/90 mmHg, and he had abdominal tenderness. The initial laboratory studies were as follows: serum urea 39.98 mmol/L (normal 0–36 mmol/L), serum creatinine 201.55 mmol/L (normal 44.2–106 mmol/L), uric acid 0.37 mmol/L (normal 0–0.33 mmol/L), potassium 3.56 mmol/L (normal 3.5–5.0 mmol/L), sodium 148 mmol/L (normal 136–145 mmol/L), pH 7.36 (normal 7.35–7.45), Hct 36.8 (normal 37–52) and total protein 64.87 g/L (normal 64–87 g/L). Urinalysis revealed only proteinuria, and daily protein excretion was 284 mg. The other biochemical parameters and blood count were normal.
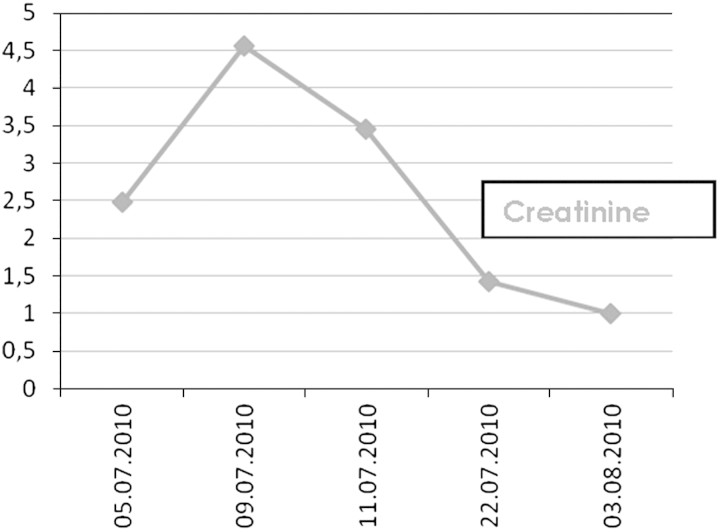
The patient was hospitalized, and the creatine supplements were discontinued. Intravenous fluids were administered. During hospitalization, his serum creatinine level increased to 403.10 mmol/L (Figure 1). Serology revealed negative antinuclear, anti-double-stranded DNA and anti-neutrophil cytoplasmic antibodies. The spiral computed tomographic scan and ultrasonography revealed no abnormalities in the kidneys.
Fig. 1.
Time course of serum creatinine level.
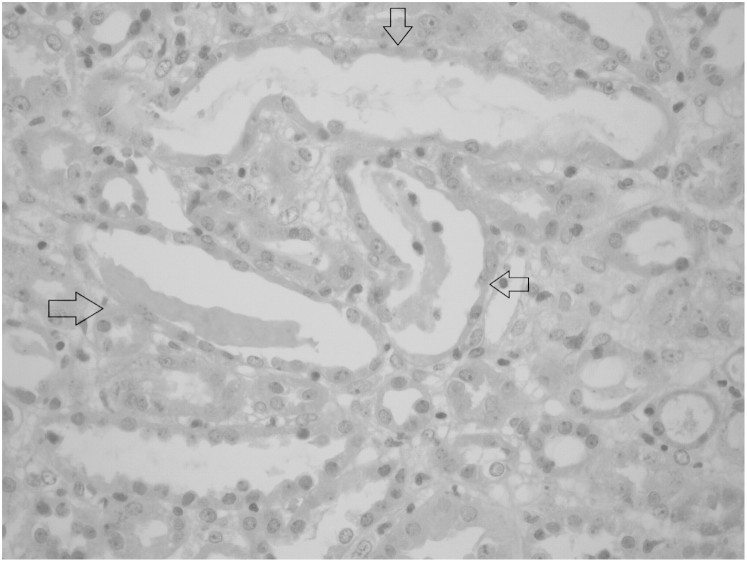
The renal biopsy revealed focal tubular injury with dilatation of tubular lumina and flattening of the tubular epithelial cells. Some of them had hyperchromatic nuclei and prominent nucleoli with occasional mitotic figures. There were sloughed epithelial cells, leucocytes and cellular debris in the tubular lumina; however, there were no pigmented casts. The glomeruli appeared to be normal. Immune complex deposition was not identified with immunoflourescence staining. With these features, the renal biopsy diagnosed acute tubular necrosis (Figure 2).
Fig. 2.
Renal biopsy showing revealed focal tubular injury with dilatation of tubular lumina and flattening of the tubular epithelial cells.
Twenty-five days after stopping the creatine supplements, the patient's blood pressure (120/70 mmHg), serum creatinine (88.4 mmol/L) and proteinuria (82 mg/day) normalized, and the patient was discharged from the hospital with a weight of 72 kg.
Discussion
Creatine monohydrate is a performance-enhancing substance. The majority (> 90%) of creatine supplementation ingested is removed from the plasma by the kidney and excreted in the urine [5].
Extensive research over the last decade has shown that oral creatine supplementation appears to be safe when used by healthy adults at recommended loading (20 g/day for 5 days) and maintenance doses (< 3 g/day), and it was largely devoid of adverse side effects [6,7]. The potential side effect of creatine supplementation, kidney damage, was based on case reports only and was associated especially with high doses of creatine supplementation or renal disease [8–10].
In our case, though the patient was using the recommended doses of creatine monohydrate, he developed renal failure. Furthermore; he also did not have a history of any renal disease or use of any nephrotoxic drugs or herbs. There is less concern today than there used to be about possible kidney damage from creatine, although there are reports of kidney damage, such as interstitial nephritis. Therefore, patients with kidney disease should avoid using this supplement. But still, some studies have shown little or no adverse impact on kidney function with the use of oral creatine supplementation [11–13].
In our patient, the renal biopsy showed acute tubular necrosis. In the literature, creatine monohydrate supplementation and ATN coexistence has not been reported previously. After stopping the creatine supplements, the patient recovered completely. He was discharged without any complaints on the 25th day.
In conclusion, it must be kept in mind that even the recommended doses of creatine monohydrate supplementation may cause kidney damage. Therefore, anyone using this supplement should be warned about this possible side effect, and the renal functions should be regularly controlled during this period.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Kreider RB, Melton C, Rasmussen CJ, et al. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol Cell Biochem. 2003;244:95–104. [PubMed] [Google Scholar]
- 2.Mayhew DL, Mayhew JL, Ware JS. Effects of long-term creatine supplementation on liver and kidney functions in American college football players. Int J Sport Nutr Exerc Metab. 2002;12:453–460. doi: 10.1123/ijsnem.12.4.453. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TM, Sewell DA, Casey A, et al. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br J Sports Med. 2000;34:284–288. doi: 10.1136/bjsm.34.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terjung RL, Clarkson P, Eichner ER, et al. American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc. 2000;32:706–717. doi: 10.1097/00005768-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar WB, Zambraski EJ. Effects of creatine use on the athlete’s kidney. Curr Sports Med Rep. 2002;1:103–106. doi: 10.1249/00149619-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bizzarini E, De Angelis L. Is the use of oral creatine supplementation safe? J Sports Med Phys Fitness. 2004;44:411–416. [PubMed] [Google Scholar]
- 7.Yoshizumi WM, Tsourounis C. Effects of creatine supplementation on renal function. J Herb Pharmacother. 2004;4:1–7. [PubMed] [Google Scholar]
- 8.Koshy KM, Griswold E, Schneeberger EE. Interstitial nephritis in a patient taking creatine. N Engl J Med. 1999;340:814–815. doi: 10.1056/NEJM199903113401017. [DOI] [PubMed] [Google Scholar]
- 9.Pritchard NR, Kalra PA. Renal dysfunction accompanying oral creatine supplements. Lancet. 1998;351:1252–1253. doi: 10.1016/s0140-6736(05)79319-3. [DOI] [PubMed] [Google Scholar]
- 10.Thorsteinsdottir B, Grande JP, Garovic VD. Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr. 2006;16:341–345. doi: 10.1053/j.jrn.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Poortmans JR, Francaux M. Adverse effects of creatine supplementation: fact or fiction? Sports Med. 2000;30:155–170. doi: 10.2165/00007256-200030030-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gualano B, Ugrinowitsch C, Novaes RB, et al. Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Eur J Appl Physiol. 2008;103:33–40. doi: 10.1007/s00421-007-0669-3. [DOI] [PubMed] [Google Scholar]
- 13.Pline KA, Smith CL. The effect of creatine intake on renal function. Ann Pharmacother. 2005;39:1093–1096. doi: 10.1345/aph.1E628. [DOI] [PubMed] [Google Scholar]