Abstract
Spina bifida (SB) is associated with chronic kidney disease as a result of vesicoureteric reflux. A proportion of patients progress to end-stage kidney disease (ESKD). Haemodialysis (HD) is probably the most common modality in ESKD, as intra-abdominal malformations and previous surgery can make peritoneal dialysis more challenging. The Chiari malformations also frequently occur in these patients. We report a case of recurrent syncope induced by HD in a patient with SB and the Chiari II malformation. Sparse data exist on the complications of HD in this patient population and on the approach to the management of dialysis-induced syncope in these individuals.
Keywords: Chiari, dialysis, mannitol, syncope
Background
The prevalence of end-stage kidney disease (ESKD) in patients with spina bifida (SB) is difficult to ascertain. A report of 283 patients with myelomeningocele treated in a single centre >25 years found chronic kidney disease in 6.7%; the rate of progression to ESKD is unclear [1]. Ninety-seven percent of patients with myelomeningocele had neuropathic bladders and upper tract dilation, and vesicoureteric reflux was more common in patients with renal dysfunction. Several reports have suggested that ESKD is the most common cause of death in SB, [2] and SB may be underappreciated as a cause of ESKD in population registries as it may be ascribed to neurogenic bladder rather than SB directly [2].
Transplantation is the treatment of choice for affected individuals [3], however, haemodialysis (HD) is often the initial modality of the renal replacement therapy chosen. Peritoneal dialysis (PD) may be challenging due to anatomical abnormalities and concerns regarding the risk of spread of infection from the peritoneal cavity to the central nervous system; however, one study found PD to be safe and effective in a paediatric cohort of PD patients with SB [2].
We report a case of recurrent syncope during HD in a man with SB and the Arnold–Chiari Type II malformation (CM II), which has been completely prevented by intravenous mannitol given pre-dialysis and intra-dialytically.
Case report
A 32-year-old man with SB developed ESKD due to reflux nephropathy and enrolled in a maintenance HD programme. After 1 year, he developed recurrent syncopal events with each HD treatment. Syncope would predictably occur 1–2 h into HD. He suffered light-headedness prior to each event and an occipital headache subsequently. He returned to normal each time over a variable period from 10 to 30 min after termination of HD. He was completely asymptomatic in the time between each HD session.
He had a ventriculo-atrial shunt due to hydrocephalus. This shunt was assessed by the neurosurgical service and found it to be functioning entirely normally. He had the cerebrospinal fluid (CSF) removed from the shunt reservoir pre-HD to increase pressure accommodation. Cardiac telemetry during the syncopal episodes demonstrated that sinus tachycardia and echocardiography were normal. Video-electroencephalography (EEG) during syncope showed a pattern consistent with the sleeping state, but no seizure activity or signs of encephalopathy.
Reducing the size of the dialysis filter, the blood flow rate and the duration of dialysis treatment in an attempt to reduce osmotic shifts were unsuccessful, however, this did reduce the severity of events and the recovery time, suggesting some benefit.
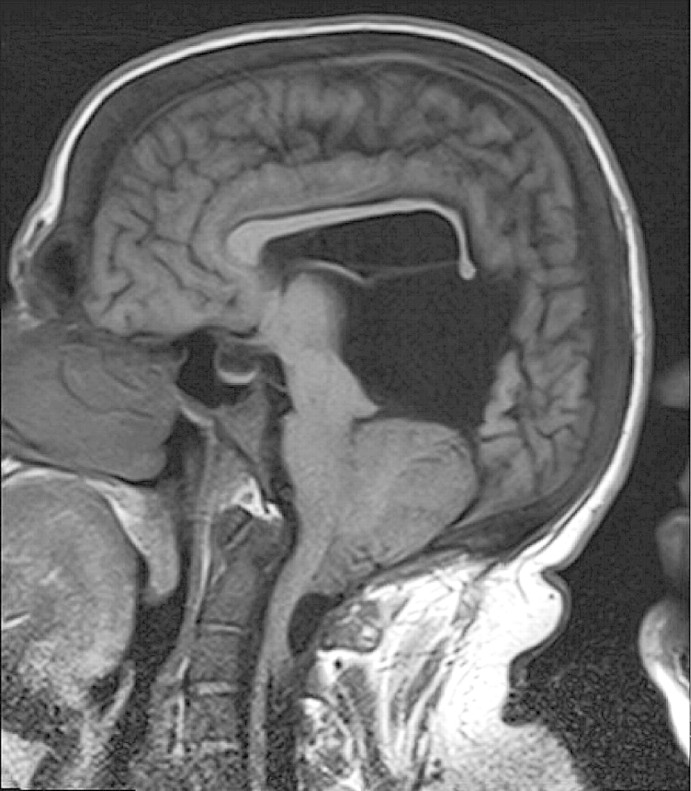
Magnetic resonance imaging (MRI) of the brain revealed CM II with severe herniation of the cerebellar tonsils through the foramen magnum, as shown in Figure 1.
Fig. 1.
MRI brain demonstrating Chiari II malformation with severe herniation of the cerebellar tonsils.
We theorize that the severity of compression of the reticular activating system was such that very minor increases in cerebral volume were enough to cause syncope and could not be compensated for by the functioning ventriculo-atrial shunt. The EEG pattern consistent with the sleeping state adds further to the evidence of compression on the reticular activating system by the herniating cerebellar tonsils.
In an attempt to reduce intra-cerebral pressure, we gave a trial of 50 g of mannitol immediately before dialysis and an additional dose at the midpoint in the treatment. This regime was immediately successful at preventing syncopal events, and he then underwent 80 HD sessions without one syncopal event. At that point, a trial of mannitol withdrawal was made, however, he again suffered a syncopal event. Mannitol was re-introduced and prevented syncope again.
Discussion
CM II is defined by downward displacement of the medulla oblongata and cerebellar tonsils through the foramen magnum and is associated with a myelomeningocele. This results in impairment of CSF circulation and hydrocephalus. Spontaneous syncope has been associated with CM I, due to compression of neurological structures by the descending tonsils. In one reported case, the recurrent syncopal events resolved after surgical decompression of the posterior fossa [4]. These syncopal episodes are most likely a result of compression of the midbrain ascending reticular system or through compression of the vertebrobasilar artery [5]. Experimental evidence suggests that CM is associated with abnormalities in control of the autonomic nervous system and may be associated with cardiac arrest due to ventricular fibrillation [6]. The association between the sudden death and CM due to compressive phenomena on the brain stem and the medulla are becoming increasingly recognized [7].
A similar case has been reported in which a woman with SB suffered recurrent syncope during HD, which was ascribed to dialysis disequilibrium syndrome (DDS) and was prevented by measures used to reduce dialysis intensity [8]. These measures did not prevent syncope in our patient. DDS is associated with cerebral oedema due to the rate of changes in osmolarity and urea removal and is directly related to dialysis intensity [9]. It is therefore reasonable to assume that changes in cerebral volume and compression of vital structures in individuals with a severe form of CM II result in syncope.
The logical first step in this situation would perhaps be to transition to PD, however, this was not possible in this case due to extensive previous abdominal surgeries. The ability of mannitol to reduce brain water and hence brain volume and intra-cranial pressure (ICP) has been demonstrated using MRI [10]. It is therefore reasonable to assume that the use of mannitol in our patient prevented syncope by reducing cerebral volume and compression of the reticular system by the herniating cerebellar tonsils. Untreated, these recurrent episodes may possibly pre-dispose to cardiac arrest.
These patients are often of small stature, which makes them more vulnerable to large fluctuations in fluid and solute concentration during dialysis, and may have low-lying cerebral venous sinuses causing elevation of intra-cranial venous pressures, which may indirectly increase ICP by reducing absorption of CSF [8].
This case sheds light on the possible complications, which may arise during HD in patients with SB and the Chiari malformation, an area where little published data exists. These difficulties may become increasingly recognized as the long-term survival in SB increases and a proportion of patients require long-term HD, intravenous mannitol may prevent syncopal events.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Torre M, Guida E, Bisio G, et al. Risk factors for renal function impairment in a series of 502 spinal dysraphisms. J Pediatr Urol. 2011;7:39–43. doi: 10.1016/j.jpurol.2010.02.210. [DOI] [PubMed] [Google Scholar]
- 2.Grunberg J, Verocay MC, Rebori A, et al. Comparison of chronic peritoneal dialysis outcomes in children with and without spina bifida. Pediatr Nephrol. 2007;22:573–577. doi: 10.1007/s00467-006-0369-y. [DOI] [PubMed] [Google Scholar]
- 3.Power RE, O'Malley KJ, Little DM, et al. Long-term followup of cadaveric renal transplantation in patients with spina bifida. J Urol. 2002;167:477–479. doi: 10.1016/S0022-5347(01)69067-0. [DOI] [PubMed] [Google Scholar]
- 4.Martinez Soto LJ, Urculo Bareno E, Ramirez Penso R, et al. [Spontaneous syncope as the only sign of Arnold-Chiari type I malformations] Neurologia. 1995;10:174–177. [PubMed] [Google Scholar]
- 5.Prilipko O, Dehdashti AR, Zaim S, et al. Orthostatic intolerance and syncope associated with Chiari type I malformation. J Neurol Neurosurg Psychiatry. 2005;76:1034–1036. doi: 10.1136/jnnp.2004.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alegre S, Garcia-Rubira JC, Patrignani G. Cardiac arrest in a 31-year-old man because of the Arnold-Chiari malformation. Int J Cardiol. 1994;46:286–288. doi: 10.1016/0167-5273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 7.Ferre Maso A, Poca MA, de la Calzada MD, et al. Sleep disturbance: a forgotten syndrome in patients with Chiari I malformation. Neurologia. 2011 doi: 10.1016/j.nrl.2011.01.008. : in press. [DOI] [PubMed] [Google Scholar]
- 8.Flannery T, Shoakazemi A, McLaughlin B, et al. Dialysis disequilibrium syndrome: a consideration in patients with hydrocephalus. J Neurosurg Pediatr. 2008;2:143–145. doi: 10.3171/PED/2008/2/8/143. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Peets AD, Hameed M, et al. Dialysis Disequilibrium Syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure—a case report. BMC Nephrol. 2004;5:9. doi: 10.1186/1471-2369-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell BA, Smith MA, Kean DM, et al. Brain water measured by magnetic resonance imaging. Correlation with direct estimation and changes after mannitol and dexamethasone. Lancet. 1987;1:66–69. doi: 10.1016/s0140-6736(87)91908-8. [DOI] [PubMed] [Google Scholar]