Abstract
Acute salicylate overdose in pregnancy is potentially fatal for both the mother and fetus and presents a unique challenge in intensive care management. While suggested thresholds exist for hemodialysis in adults with toxic salicylate ingestion, it is unclear if these thresholds remain appropriate for the gravid patient, particularly given that medications such as acetylsalicylic acid may cross the placental barrier and accumulate in the fetal bloodstream. We describe a case of a gravid patient at ∼37 weeks gestational age with a self-reported acetylsalicylic acid ingestion of 32.5 g and review prior cases of both acute and chronic salicylate ingestion in pregnancy in order to determine the clinical precedent for hemodialysis in this situation.
Keywords: acetylsalicylic acid, hemodialysis, overdose, pregnancy, salicylate toxicity
Case report
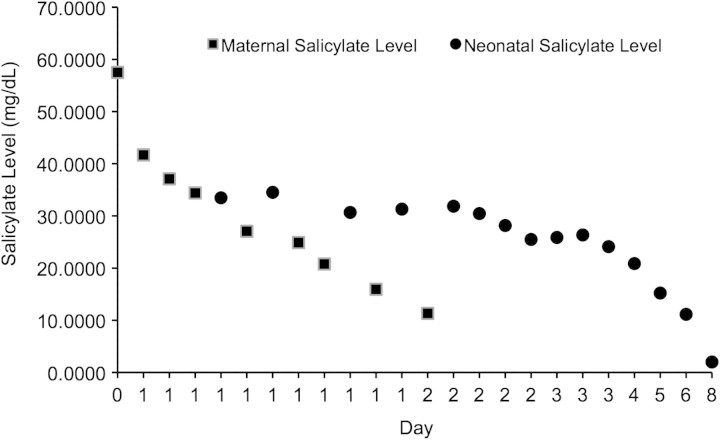
A 19-year-old, gravida 1, para 0 woman at 39 weeks gestation with a past medical history notable for mild mental retardation, bipolar disorder and hypothyroidism presented to an outside hospital with bloody emesis and tinnitus after a self-reported ingestion of 100 tablets (32.5 g) of aspirin several hours prior. Upon initial presentation, she was agitated and tachypneic, with a respiratory rate of 40 breaths/minute and tachycardic, with a heart rate of 120–130 beats/minute, but otherwise hemodynamically stable. Her laboratory data were notable for a salicylate level of 57.5 mg/dL, bicarbonate of 16 mmol/L and an anion gap of 14. An ultrasound showed a fetal heart rate of 160–170 beats/minute. The patient was given bicarbonate and glucose and transferred to the medical intensive care unit, where she was continued on a bicarbonate drip for urine alkalinization. A repeat salicylate level was 41.69 mg/dL with an arterial pH of 7.60. She remained persistently alkalotic due to the bicarbonate drip. Coagulation studies, including prothrombin time (PT), activated partial thromboplastin time (PTT) and international normalized ratio (INR), were all within normal limits. Her hematocrit was 37% and platelets were 487 000/mm3. The patient remained confused and agitated and continued to complain of tinnitus. A series of her serum salicylate levels is shown in Figure 1.
Fig. 1.
Maternal and neonatal acetylsalicylic acid levels over time
As the threshold for hemodialysis for aspirin overdose at our institution is a serum salicylate level of 100 mg/dL, the patient was determined not to be a candidate for hemodialysis. Her serum salicylate level continued to decrease with intravenous fluids and bicarbonate therapy. A fetal ultrasound indicated a gestational age of 37–38 weeks (by fetal biparietal diameter and femur length). The fetal heart tracing showed a baseline fetal heart rate of 160–170 beats/minute with minimal variability and multiple spontaneous decelerations. The patient’s medical and mental status continued to improve. She also began to contract regularly, with cervical change to 3 cm dilation, consistent with early labor. The fetal heart tracing continued to be non-reassuring. Given the non-reassuring fetal heart tracing and the signs that the patient was entering early labor, she was extensively counseled as to the risks and benefits of a Cesarean delivery for both herself and the fetus and she consented to the procedure. To minimize risk of maternal hemorrhage, she was given a platelet transfusion prior to transfer to the operating room. A second unit of platelets was also started as the patient was taken to the operating room for an emergent Cesarean section under general anesthesia. A vertical skin incision, rather than a Pfannenstiel incision, was used to decrease the bleeding risk. Thick meconium was noted at the time of delivery, but the procedure was otherwise uncomplicated. Blood loss was estimated at 700 cc.
The female neonate weighed 3100 g at delivery and was initially limp and apneic with thick meconium-stained fluid. Apgars were 1 and 7 at 0 and 5 min, respectively. The initial heart rate was <60 beats/minute and chest compressions were not performed; the neonate was subsequently intubated. Arterial and venous cord gases drawn immediately after delivery were pH 7.32, CO2 43.6 mmHg, base excess −4 and pH 7.36, CO2 36.6 mmHg, and base excess −4.3, respectively. A salicylate level also drawn at this time was 33.5 mg/dL, while a level drawn simultaneously from the mother was 27.06 mg/dL. Initial complete blood count and coagulation studies at 1 h of life revealed a white blood cell count of 23 900 cells/mm3, hemoglobin 13.6 mmol/L, hematocrit 40.3%, platelets 285 000/mm3, PT 26.5, PTT 41.6, INR 2.4 and fibrinogen 169. Electrolytes drawn at ∼6 h of life were as follows: sodium 137 mEq/L, potassium 2.8 mEq/L, chloride 101 mmol/L, bicarbonate 17 mmol/L and calcium 7.1 mEq/L. A urine toxicology screen was negative for amphetamines, barbituates, benzodiazepines, cannabinoids, cocaine, methadone, opiates and oxycodone. Meconium toxicology screen returned positive for cannabinoids. A head ultrasound was performed and showed no intracranial hemorrhage and no anomalies or malformations. A chest X-ray also performed shortly after delivery was suggestive of meconium aspiration syndrome. The neonate received surfactant via endotracheal tube and was started on intravenous fluids with supplemental bicarbonate. She also received one transfusion of fresh frozen plasma (10 cc/kg) and platelets (10 cc/kg) due to the increased INR and mild oozing noted with placement of the umbilical artery and venous catheters. Ampicillin and gentamicin were started empirically for a 48-h course; blood cultures drawn prior to antibiotic therapy showed no growth after 48 h. She was extubated ∼17 h after delivery and remained stable. The post-natal course was remarkable for respiratory alkalosis, initial hypokalemia and hypocalcemia that resolved following repletion and normoglycemia. Neonatal salicylate levels are also shown in Table 1. The neonate did not pass a hearing test performed on day of life two; the results of further testing are unknown.
Discussion
There have been several prior reports of in utero salicylate toxicity, with three involving acute ingestion [1–3], two of which resulted in intrauterine fetal demise [2, 3]. The remaining cases involve chronic daily usage in the last trimester of pregnancy, and in two of such cases [4, 5], fetal or neonatal death was the result, one in utero [4] and one at day of life nine [5]. In several cases, the diagnosis of in utero salicylate exposure was made retrospectively and post-partum [1, 5–8]. In those cases in which salicylate exposure was known in the antepartum setting, the outcome was either intrauterine fetal demise [2–4] or Cesarean section due to fetal distress [9, 10], although the latter two cases involved chronic salicylate exposure during pregnancy rather than acute intoxication. The treatment in these cases of known in utero salicylate toxicity has largely been expectant management, with outcomes ranging from fetal demise in utero in the cases of large-volume ingestion [2–4] to delivery without sequelae, primarily in the cases of chronic ingestion [6, 7, 10, 11] although not exclusively [1]. Therefore, in acute maternal toxic salicylate ingestion, appropriate treatment is unclear, particularly whether or not dialysis or prompt delivery would be beneficial in preventing fetal demise in utero.
In adult patients, the threshold for hemodialysis to further counteract the effects of salicylates has been suggested at 70–80 [12] and 100 mg/dL at our institution. However, it has been noted that the severity of the intoxication does not always correlate with serum salicylate levels [13]. While neither our case nor prior cases have met these criteria, the three cases involving fetal demise in utero involved maternal salicylate levels in the 50–60 mg/dL range [3, 4], and it has been shown that fetal blood levels are ∼1.5 times maternal levels [9, 11]. Furthermore, the neonate eliminates salicylate more slowly due to immature glucuronidation and renal excretory pathways [9, 11], and salicylate tends to concentrate in the fetal brain due to a smaller intravascular/intracellular pH gradient in the fetus [4]. The intracerebral accumulation of salicylates is a major cause of morbidity and mortality. This raises the question of whether dialysis should be initiated at lower levels in cases of salicylate toxicity in pregnancy, and if this might be beneficial for the fetus. In only one prior report was hemodialysis utilized [4], and this was initiated in a patient who presented after in utero fetal demise had already occurred.
To our knowledge, there have been no reports of the use of dialysis for toxic overdose of any drug in pregnant patients. There has been a study of acute kidney injury during pregnancy requiring dialysis [14], but fetal outcomes were not followed. In our case, a gravid patient presented with initial salicylate levels below those in prior case reports where significant morbidity or mortality was the outcome. Although the fetus showed signs of distress upon presentation, the decision was made to delay delivery until maternal status had been optimized. It was unclear whether the benefits of maternal hemodialysis would be conferred upon the fetus, as hemodialysis would have little effect on the salicylate that had already entered fetal circulation. In addition, fetal distress in this situation could be attributed to derangements in fetal acid–base status, as mentioned in previous case reports [4], with fetal acidosis persisting despite our patient’s alkalosis due to intravenous bicarbonate therapy. Indeed, it has been hypothesized that maternal hemodialysis does not benefit the fetus in the case of acute salicylate overdose and that emergent delivery is the most beneficial for the fetus at risk for in utero salicylate toxicity at or near term [4]. Maternal hemodialysis was not initiated in our case, with no adverse effect on fetal outcome. As shown in Figure 1, neonatal salicylate levels, although higher than maternal levels, also declined spontaneously within 24 h of life.
As the patient’s condition stabilized without hemodialysis, she spontaneously began to labor and mode of delivery became a concern. The fetal heart tracing continued to show signs of fetal distress, and despite progressive cervical change, it was thought that the fetus would not tolerate a vaginal delivery. As salicylate metabolites accumulate in the fetal brain [5], potentially fatal intracerebral hemorrhage could result, as has been reported in other cases [2, 8]. Given these concerns for fetal well-being, a Cesarean section was determined to be the optimal mode of delivery.
Our experience with this case and further research on similar situations illustrates that maternal hemodialysis is of little benefit to the fetus in cases of salicylate ingestion and that expectant management is reasonable until maternal condition stabilizes, at which time, the patient should be counseled for a Cesarean section to avoid fatal fetal intracerebral hemorrhage.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Earle R., Jr Congenital salicylate intoxication—report of a case. N Engl J Med. 1961;265:1003–1004. doi: 10.1056/NEJM196111162652009. [DOI] [PubMed] [Google Scholar]
- 2.Jackson AV. Toxic effects of salicylate on the fetus and mother. J Pathol Bacteriol. 1958;60:587–593. [Google Scholar]
- 3.Rejent TA, Baik S. Fatal in utero salicylism. J Forensic Sci. 1985;30:942–944. [PubMed] [Google Scholar]
- 4.Palatnick W, Tenenbein M. Aspirin poisoning during pregnancy: increased fetal sensitivity. Am J Perinatol. 1998;15:39–41. doi: 10.1055/s-2007-993896. [DOI] [PubMed] [Google Scholar]
- 5.Govaert P, Staelens V, Vanhaesebrouch P. Perinatal intracranial hemorrhage due to maternal salicylate ingestion. Clin Pediatr (Phila) 1995;34:174–175. doi: 10.1177/000992289503400317. [DOI] [PubMed] [Google Scholar]
- 6.Buck ML, Grebe TA, Bond GR. Toxic reaction to salicylate in a newborn infant; similarities to neonatal sepsis. J Pediatr. 1993;122:955–958. doi: 10.1016/s0022-3476(09)90027-0. [DOI] [PubMed] [Google Scholar]
- 7.Lynd PA, Andreasen AC, Wyatt RJ. Intrauterine salicylate intoxication in a newborn. Clin Pediatr (Phil) 1976;15:912–913. doi: 10.1177/000992287601501010. [DOI] [PubMed] [Google Scholar]
- 8.Karlowicz MG, White LE. Severe intracranial hemorrhage in a term neonate associated with maternal acetylsalicylic acid ingestion. Clin Pediatr (Phila) 1993;32:740–743. doi: 10.1177/000992289303201206. [DOI] [PubMed] [Google Scholar]
- 9.Garrettson LK, Procknal JA, Levy G. Fetal acquisition and neonatal elimination of a large amount of salicylate. Clin Pharmacol Ther. 1975;17:98–103. doi: 10.1002/cpt197517198. [DOI] [PubMed] [Google Scholar]
- 10.Ahlfors CE, Shwer ML, Ford KW. Bilirubin-albumin binding in neonatal salicylate intoxification. Dev Pharmacol Ther. 1982;4:47–60. doi: 10.1159/000457390. [DOI] [PubMed] [Google Scholar]
- 11.Levy G, Procknal JA, Garrettson LK. Distribution of salicylate between neonatal and maternal serum at diffusion equilibrium. Clin Pharmacol Ther. 1975;18:210–214. doi: 10.1002/cpt1975182210. [DOI] [PubMed] [Google Scholar]
- 12.Hill JB. Salicylate intoxication. N Engl J Med. 1973;288:1110–1113. doi: 10.1056/NEJM197305242882107. [DOI] [PubMed] [Google Scholar]
- 13.Fertel BS, Nelson LS, Goldfarb DS. The underutilization of hemodialysis in patients with salicylate poisoning. Kidney Int. 2009;75:1349–1353. doi: 10.1038/ki.2008.443. [DOI] [PubMed] [Google Scholar]
- 14.Silva GB, Jr, Monteiro FA, Mota RMS, et al. Acute kidney injury requiring dialysis in obstetric patients: a series of 55 cases in Brazil. Arch Gynecol Obstet. 2009;279:131–137. doi: 10.1007/s00404-008-0682-8. [DOI] [PubMed] [Google Scholar]