Abstract
The elimination of metformin is exclusively through the kidneys and elevated plasma concentrations can cause lactic acidosis. We report a case of severe lactic acidosis (pH 6.60) occuring with ostensibly normal therapeutic doses of metformin in the setting of acute renal failure. Continuous veno-venous haemodiafiltration decreased plasma metformin concentrations from 266 lmol/L at presentation to 68 lmol/L, 21 h later. The patient improved rapidly.
Keywords: lactic acidosis, metformin
Background
Metformin is widely used for the treatment of type 2 diabetes mellitus. It is eliminated exclusively through the kidneys, mostly by tubular secretion. Elevated plasma metformin may cause lactic acidosis, a rare but potentially fatal side effect. It has been shown that plasma metformin concentrations are only slightly increased in patients with impaired renal function down to an estimated glomerular filtration rate (eGFR) of 30 mL/min/1.73m2 [1].
Case report
A 55-year-old woman with diabetes mellitus type 2 presented to the emergency department (ED) with somnolence. She had consulted her family physician 1 day prior to this with a 3-week history of vomiting and fatigue. Laboratory investigations at this stage revealed a plasma creatinine of 1057 μmol/L compared to 115 μmol/L (eGFR 42 mL/min/1.73m2 according to Modification of Diet in Renal Disease formula) 6 months earlier. Her medications included metformin 1000 mg three times a day; simvastatin 20 mg once a day, atenolol 50 mg once a day and ramipril 10 mg once a day. The following day, the physician tried to contact the patient and when she did not answer the phone, ambulance personnel entered her apartment with the help of the police.
On ED presentation, she was found to be semi-conscious with a Glasgow Coma Score of 8. Her respiratory rate was 30 breaths/min, non-invasive blood pressure was immeasurable, invasive blood pressure was 45/30 mmHg, heart rate was 85 beats/min and urinary bladder temperature was 31.2°C. There were peaked T waves on the electrocardiogram. Initial arterial blood gas analysis showed a profound acidosis, with a pH of 6.60 (reference range 7.37–7.47), arterial partial pressure of carbon dioxide (PaCO2) 2.8 kPa (reference range 4.6–6.0 kPa), arterial partial pressure of oxygen (PaO2) 14.9 kPa (reference range 8.0–13.0 kPa) measured while the patient received 10 L/min O2 via resuscitation mask, base excess −29 mmol/L (reference range −3 to +3 mmol/L), a high lactate concentration of 20.0 mmol/L, a plasma creatinine of 1205 μmol/L and an increased plasma potassium concentration of 8.4 mmol/L. The anion gap was 37.4 mmol/L.
The patient was admitted to the intensive care unit (ICU) and treated with intravenous (i.v.) fluids, norepinephrine and dobutamin i.v. infusions (0.25 and 5.1 μg/kg/min, respectively), i.v. calcium chloride (180 mg), i.v. sodium bicarbonate (540 mmol), beta agonist inhalation and an i.v. loop diuretic. Continuous veno-venous haemodiafiltration (CVVHDF) was commenced and maintained for 21 h. Endotracheal intubation was not performed since she had by this time adequate airway and respiratory status.
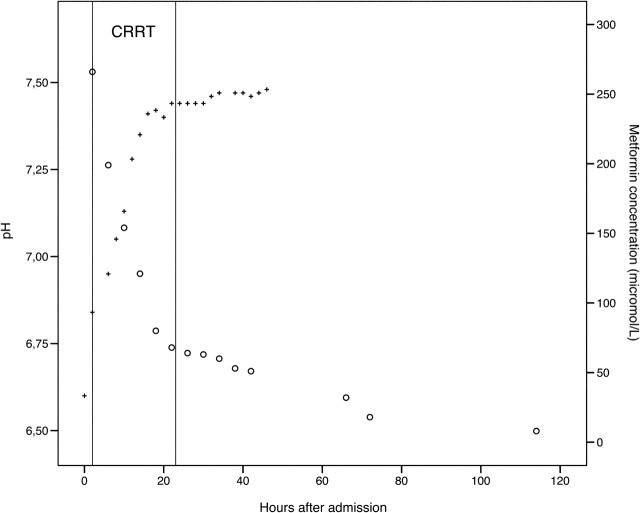
At ICU admission, the plasma metformin was 266 μmol/L (therapeutic range <20 μmol/L), decreasing to 68 μmol/L at the end of the CVVHDF treatment (Figure 1). Thereafter, it slowly declined to reach normal therapeutic values after 3 days. The elimination half-time for metformin was ∼10 h during CVVHDF and ∼ 29 h after its cessation.
Fig. 1.
Serum metformin levels and pH during and after CRRT (continuous renal replacement therapy). Therapeutic serum concentrations recommended to be <20 μmol/L.
The patient improved rapidly and was discharged to a medical ward after 45 h in the ICU. Plasma creatinine improved but remained elevated after 5 weeks, 156 μmol/L, with an eGFR of 27 mL/min/1.73m2. Metformin was discontinued and plasma glucose was normal with repaglinide 5 mg three times a day.
Discussion
Lactic acidosis is an uncommon but severe side effect of metformin treatment. The mechanism by which metformin causes raised lactate in the blood is unclear but it is suggested that metformin blocks the conversion of lactate and pyruvate to glucose giving rise to anaerobic acidosis (type B). Lactic acidosis is commonly associated with acute metformin intoxication. However, as demonstrated in this case, extreme lactic acidosis may develop in patients on therapeutic doses of metformin in the setting of acute renal failure induced by a prolonged episode of dehydration. In this patient, the pre-morbid history of moderately impaired renal function and angiotensin-converting enzyme inhibitor treatment may have contributed to acute renal failure. Lemyze et al. reported a very similar patient earlier. These authors also performed renal replacement therapy and measured metformin levels in their patient. They made the interesting observation that CVVHF was not enough to lower metformin levels. The levels fell only if dialysis was done or if CVVHF was augmented by dialysis CVVHDF. Our findings confirm this earlier study [2].
Metformin is eliminated through renal excretion and reduced renal function will lead to accumulation of the drug. Plasma levels will only increase marginally down to a glomerular filtration rate of 30 mL/min/1.73m2 [1], probably because tubular secretion is a prominent mechanism for excretion. When renal function is further lowered, the plasma concentration of metformin will rise rapidly if the patient continues to take the drug. Metformin in plasma is a non-protein-bound water-soluble substance with low-molecular mass and is quickly cleared by haemodialysis. In the present case, the patient received treatment with CVVHDF for ∼21 h, which reduced the plasma metformin to 1/5 of the initial concentration. Conventional acute haemodialysis would probably have eliminated metformin more efficiently than CVVHDF, but in this case, the patient’s profound haemodynamic instability led us to choose CVVHDF as the treatment of choice.
This patient also received a loop diuretic. Theoretically and experimentally, loop diuretics may lessen the risk of hypoxic injury to the cells of the loop of Henle. This has not, however, been convincingly shown in clinical studies [3] and the risk of ototoxicity should be considered. The use of loop diuretic in our case may therefore be questioned, especially in the presence of dehydration-induced acute renal failure.
Although CVVHDF also corrects acidosis, we chose to correct the metabolic acidosis simultaneously with intravenous infusions of sodium bicarbonate. While bicarbonate therapy for lactic acidosis has been described previously [4], one may appropriately question the wisdom of giving bicarbonate in this situation, given that bicarbonate therapy is not without risk. Our rationale was that extreme acidosis and hyperkalaemia were probable causes of circulatory shock, and the treating physician was concerned about the risk of imminent cardiac arrest and malignant arrhythmias, both of which would have been difficult to treat in the setting of severe acidosis. Nevertheless, the use of sodium bicarbonate in this setting is controversial and may potentially worsen intracellular acidosis, particularly in the setting of impaired ventilation. Other complications such as hypokalaemia, increased carbon dioxide production, hyperosmolality, volume overload and overshoot metabolic alkalosis, have also been described [5].
Metformin-associated lactic acidosis has been defined as a lactate value >5 mmol/L and a bicarbonate level <22 mmol/L in a patient on metformin medication or after metformin overdose [6]. Determination of plasma metformin may be useful to differentiate metformin-induced lactic acidosis from other causes. If ketoacidosis and uraemia can be excluded, then intoxication with exogenous agents should be considered and metformin should be kept in mind [7].
In clinical practice, we have noted that p-metformin always exceeds 250 μmol/L in cases with severe lactic acidosis (pH < 7.2). This level is 10–15 times higher compared to therapeutic concentrations of metformin [1].
To conclude, we have presented a case with severe lactic acidosis successfully treated with supportive care and continuous haemodiafiltration. Physicians starting treatment with metformin should assess renal function and instruct the patient to stop taking the drug and consult their physician in any situation where dehydration may occur. Metformin intoxication should be suspected even with ostensibly normal doses in the setting of acute renal impairment.
Acknowledgments
The authors thank Michelle S. Chew, Associate Professor at the Department of Intensive Care Medicine, Skåne University Hospital Malmö, for reviewing and providing helpful feedback during the manuscript development process.
Conflict of interest statement. None declared.
References
- 1.Frid A, Sterner GN, Londahl M, et al. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care. 2010;33:1291–1293. doi: 10.2337/dc09-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemyze M, Baudry JF, Collet F, et al. Life threatening lactic acidosis. BMJ. 2010;340:c857. doi: 10.1136/bmj.c857. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Delaney A, Haase M, et al. Loop diuretics in the management of acute renal failure: a systematic review and meta-analysis. Crit Care Resusc. 2007;9:60–68. [PubMed] [Google Scholar]
- 4.Heaney D, Majid A, Junor B. Bicarbonate haemodialysis as a treatment of metformin overdose. Nephrol Dial Transplant. 1997;12:1046–1047. doi: 10.1093/ndt/12.5.1046. [DOI] [PubMed] [Google Scholar]
- 5.Kraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol. 2010;6:274–285. doi: 10.1038/nrneph.2010.33. [DOI] [PubMed] [Google Scholar]
- 6.Peters N, Jay N, Barraud D, et al. Metformin-associated lactic acidosis in an intensive care unit. Crit Care. 2008;12:R149. doi: 10.1186/cc7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CT, Chen YC, Fang JT, et al. High anion gap metabolic acidosis in suicide: don't forget metformin intoxication—two patients' experiences. Ren Fail. 2002;24:671–675. doi: 10.1081/jdi-120013973. [DOI] [PubMed] [Google Scholar]