Introduction
In the February issue of NDT Plus, Thomson et al. [1] reported on a case of nosocomial transmission of hepatitis C virus (HCV). The new infection was picked up through routine screening in September 2009 and the likely source patient was identified using molecular virology. A retrospective analysis revealed that while the source and case patients were normally treated in different units, there was one occasion in Spring 2009 when the source patient dialysed on the morning shift and the case patient dialysed on the same machine in the afternoon. As audits of the implementation of infection control procedures had not revealed any problems in the renal unit, the authors of the report made the assumption that ‘transmission of HCV via the haemodialysis circuit is the most plausible explanation of this event’. Based on this event, the authors recommended the use of dedicated machines in the HCV-positive population.
As Thomson et al. points out, this recommendation goes beyond the current guidance provided by Kidney Disease: Improving Global Outcomes (KDIGO), [2], European Renal Best Practice [3] and the UK Renal Association [4]. None of these bodies advocate the use of dedicated machines. It is acknowledged that it may be considered necessary to isolate HCV-positive patients in units with a high prevalence or in cases of repeated nosocomial transmission despite attempts to ensure rigorous use of hygienic precautions. However, in these circumstances, the HCV-positive population should be treated in a separate location and by dedicated staff who do not move between infected and uninfected patients. Such isolation policies require additional resources and complicated arrangements for patients with unknown virus status [5].
The policy adopted in response to the case reported by Thomson et al. is to ensure that all HCV-positive patients use dedicated haemodialysis machines but ‘not’ to physically isolate the HCV-positive population from the HCV-negative population. This strategy makes sense only if the assumption that transmission of HCV occurred via the haemodialysis circuit is correct.
Assessment of the risk of transmission via the haemodialysis circuit
A discussion group was formed within the UK Association of Renal Technologists (ART) to assess the risk of transmission of HCV via the haemodialysis circuit. There are two fluid circuits to consider. The first is the dialysate circuit. In a ‘single pass’ machine (where the dialysate passes through the dialyser only once then goes to the drain), it is practically impossible for a blood-borne virus to pass from one patient to another via the dialysate. The virus is 40–60 nm in diameter, while the pores in a dialyser membrane are <10 nm across [6]. So the dialyser used for the infected patient would have to be defective in a way that produced pores large enough for the virus to pass through but not large enough to cause a detectable blood leak. After passing through the membrane, the virus would have to resist being flushed to drain and find a way to the part of the circuit where fresh dialysate is transferred to the dialyser before the next patient was connected. Another defective dialyser would be required, only now the extra large pores would have to be in a region where there is ‘back filtration’ (fluid transfer from the dialysate to the blood). It is hard to imagine how sufficient HCV to cause infection could enter the blood in this way.
A more plausible route for the virus to get into the fresh dialysate would be through contamination of the connector between the dialysate hose and the dialyser from a blood spillage onto the dialyser that was not effectively cleaned up. However, to infect the next patient, the virus would have to avoid being flushed away during prime and it would still have to cross the dialyser membrane against the ultrafiltration flow. Contamination of the connectors through blood spillage is much more likely to result in cross-infection if the blood that has not been removed is transferred to the hands or gloves of the staff.
The second haemodialysis circuit is the blood circuit. As the lines and dialyser are removed between patients, the only possible route of transmission via this circuit is by contamination of the pressure monitoring devices where the lines connect to the machine. Transmission via this route occurred during the devastating outbreaks of hepatitis B in dialysis units in the 1970s and led to the mandatory incorporation of hydrophobic filters (‘transducer protectors’) to prevent the pressure monitoring ports from being flooded with blood. If blood tracks up the lines to the transducer protector, it can breach the filters and contaminate the pressure port. The KDIGO and UK Renal Association guidelines warn that this can happen and recommend that staff inspect the transducer protectors and take the machine out of service if there is evidence that blood has passed through the filter and may have entered the machine. Cross-infection could occur if blood breached the transducer protectors in two consecutive sessions and enough HCV to cause infection was able to cross from the contaminated port back through the filter and into the bloodline. This method of transmission was put forward by Sartor et al. [7] as a possible explanation of a seroconversion in 2001. The interior of the pressure port is not disinfected with the internal fluid pathways and is inaccessible when cleaning the exterior of the machine, so evidence of blood that breaches the transducer protectors will remain until the port is cleaned or replaced during servicing or repair.
Alternative transmission routes
The evidence to show that transmission of HCV is more likely to occur through failures of hygienic precautions, including contamination of the environment (with subsequent transfer onto hands or gloves), than through the haemodialysis circuit is summarized in the KDIGO guidelines [2]. Twenty published outbreak investigations where molecular virology had been used to identify the source and case patients were reviewed. The authors of all 20 studies were unable to conclusively establish the specific transmission route(s), but all considered breaches in infection control, including failure to decontaminate pressure ports in one case, to be the probable cause of the outbreak. In 18 of 20 studies, the authors reported that some or all patients with new HCV infection had never shared the dialysis machine with the source patient either because they dialysed at the same time or because the unit policy was to assign HCV-positive patients to separate machines.
It is clear from the literature that the precise route of transmission in cases of nosocomial infection is rarely, if ever, established. The investigations are usually carried out months after the infection took place and it is not usually possible to pinpoint the exact session when transmission occurred. In the absence of a real culprit, the machine is often assumed to be the route of transmission. The assessment carried out by ART shows that risk of transmission via the haemodialysis circuit is very low and could be eliminated completely if blood ingress into the pressure port is prevented. Contamination of the exterior surface of the machine is certainly a feasible route of transmission as is contamination of the chair, blood pressure monitors and any other equipment at the dialysis station. However, if external contamination is the problem, the use of dedicated machines may be counter-productive especially if it requires staff to move machines between shifts as their time would be better spent cleaning the exterior of the machine that is in place and the other surfaces at the station. Technical staff soon become aware of weak points in cleaning protocols when repairing and servicing equipment and can provide feedback to help minimize the risk of cross-infection.
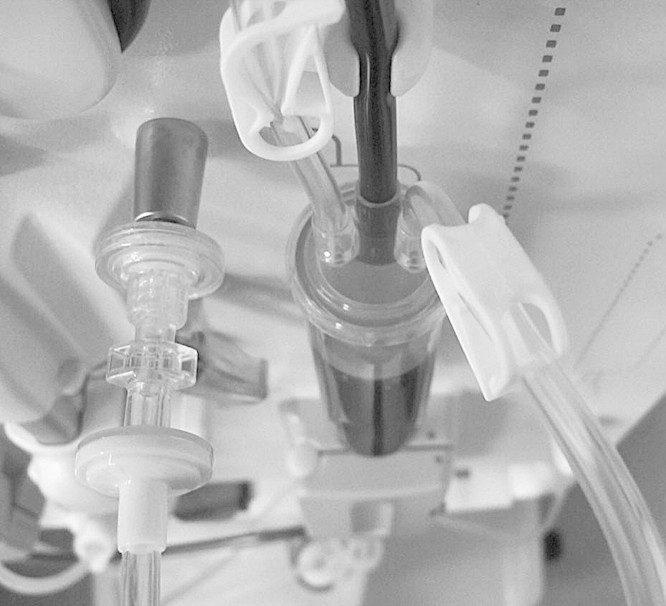
The use of a second transducer protector in series with the standard ones on the blood lines for patients who are HCV-positive (as described in the rationale to guideline 2.4 of ref. 4 and shown in Figure 1) is the ideal measure for eliminating the risk of transmission via the pressure ports. As the use of double transducer protectors means that HCV-positive patients can share machines with uninfected patients, they can dialyse in any unit and on any shift, and patients who have hepatitis B immunity do not require named machines when returning from holidays in high-risk areas. In addition, breaches of the filters in both transducer protectors will occur very rarely so machines will not have to be taken out of service as often.
Fig. 1.
Double transducer protectors on the venous pressure monitoring line.
Summary
Based on this review of the plausibility of transmission of HCV via the haemodialysis circuit, the ART discussion group could see no merit in the use of dedicated machines. The group considers the current national, European and international guidelines for prevention of transmission of HCV to be appropriate in terms of both patient care and equipment management.
Teaching points
(1)The risk of HCV transmission via the haemodialysis machine circuits is very low and can be eliminated by using double (in-series) transducer protectors.
(2)The response to unexplained seroconversion(s) should focus on the probable routes of transmission, i.e. contamination of hands, gloves and equipment through failure to implement strict hygienic precautions.
(3)The technical staff responsible for repairing and servicing equipment used in dialysis units should be asked to provide feedback on the weak points in cleaning protocols and to record contamination of the pressure ports.
(4)If nosocomial transmission occurs despite attempts to ensure rigorous use of hygienic precautions, the HCV-positive population should be treated in a separate location and by dedicated staff as advocated by the current guidelines.
Acknowledgments
The group thanks Isabel Brown and Peter Thomson from Glasgow Royal Infirmary and Duncan Ferguson from Health Facilities Scotland, for their contributions to the discussion.
Conflict of interest statement: None declared.
References
- 1.Thomson PC, Williams C, Aitken C, et al. A case of hepatitis C virus transmission acquired through sharing a haemodialysis machine. Nephrol Dial Transplant Plus. 2011;4:32–35. doi: 10.1093/ndtplus/sfq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Guideline 3: preventing HCV transmission in hemodialysis units. Kidney Int. 2008;73(Suppl 109):S46–S52. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 3.European Best Practice Guidelines for Haemodialysis (Part 1). Guideline VI.6: prevention and management of HBV, HCV and HIV in HD patients. Nephrol Dial. Transplant. 2002;17(Suppl 7):78–81. [Google Scholar]
- 4.Geddes C, Lindley E, Duncan N. Renal association clinical practice guideline on prevention of blood borne virus infection in the renal unit. Nephron Clin Pract. 2011;118(Suppl 1):C165–C188. doi: 10.1159/000328068. [DOI] [PubMed] [Google Scholar]
- 5.Karkar A, Abdelrahman M, Ghacha R, et al. Prevention of viral transmission in HD units: the value of isolation. Saudi J Kidney Dis Transpl. 2006;17:183–188. [PubMed] [Google Scholar]
- 6.Fabrizi F, Martin P. Transmission of hepatitis C virus in the hemodialysis setting. Hot Topics in Viral Hepatitis. 2008;11:13–18. [Google Scholar]
- 7.Sartor C, Brunet P, Simon S, et al. Transmission of hepatitis C virus between hemodialysis patients sharing the same machine. Infect Control Hosp Epidemiol. 2004;25:609–611. doi: 10.1086/502448. [DOI] [PubMed] [Google Scholar]