Abstract
Coupled plasma filtration adsorption (CPFA) is an extracorporeal blood purification therapy based on non-specific pro- and anti-inflammatory mediator adsorption on a special resin cartridge coupled with continuous veno-venous haemofiltration or continuous veno-venous haemodiafiltration and is one of the emerging treatments for septic patients. However, in the literature, there are limited data about its efficacy in treating patients with acute diseases but without the traditional criteria for sepsis. We describe the case of a 43-year-old male who developed acute respiratory distress syndrome secondary to pneumonia and acute kidney injury, whose clinical conditions rapidly improved after early CPFA therapy.
Keywords: acute kidney injury, acute respiratory distress syndrome, coupled plasma filtration adsorption, pneumonia, cytokines
Background
Coupled plasma filtration adsorption (CPFA) is an extracorporeal blood purification therapy that uses a plasma filter to separate plasma from the blood, a sorbent cartridge that removes various substances from the separated plasma and a haemofilter. The aim of this treatment is to reduce the levels of inflammatory mediators in patients with acute inflammatory diseases [1, 2]. This technique has been proposed as a blood purification therapy in the course of sepsis and multiple organ dysfunction syndrome [3]. However, as yet, its role in treating patients with acute diseases but without the traditional criteria for sepsis has not been defined. In this case report, we describe the potential usefulness of CPFA in association with conventional drug therapy in treating a patient with acute kidney injury (AKI) and acute respiratory distress syndrome (ARDS), possibly mediated by the removal of pro-inflammatory cytokines.
Case report
A 43-year-old male was admitted to our Nephrology Unit for dyspnoea and fever. A biopsy diagnosis of membranous glomerulonephritis with chronic kidney disease not requiring dialysis (estimated glomerular filtration rate of 18 mL/min, serum creatinine 7.1 mg/dL) had been made in our unit 3 months earlier. The patient was in treatment with cyclophosphamide (1 mg/kg PO daily) and prednisone (0.5 mg/kg PO daily). Ten days prior to the present hospitalization, his serum creatinine was 5.0 mg/dL. At the time of admission, the patient was dyspnoic, disoriented, tachycardic (110 beats/min) and febrile (37.8°C) with an oxygen saturation of 86.7% while breathing room air. Chest auscultation revealed scattered, coarse crepitations and rubbing at the right side of the chest and diminished air entry into both lung bases. The main clinical and laboratory data are summarized in Table 1. An arterial blood gas (ABG) test showed the presence of Type 1 acute respiratory failure. Laboratory investigations revealed lymphocytopoenia and a deterioration of renal function. The Acute Physiology And Chronic Health Evaluation (APACHE) II scored 18. Blood cultures and tests for pneumotropic microbial agents (Cytomegalovirus, Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae, Epstein-Barr Virus and Adenovirus) were negative. Sputum culture was positive for Candida albicans. An antimicrobial therapy with piperacillin/tazobactam, levofloxacin and fluconazole was started. Approximately 48 hours after hospitalization, ABG test while breathing through a Venturi mask (FiO2 = 0.4) confirmed the presence of chronic metabolic acidosis, with a compensatory respiratory alkalosis. A high-resolution-computed tomography (HRCT) scan of the chest showed bilateral patchy areas of ground-glass consolidation (Figure 1A). A diagnosis of ARDS was made and continuous positive airway pressure (CPAP) ventilation was started. The worsening of respiratory function was followed by the onset of atrial fibrillation and by the further increase of serum creatinine levels (8 mg/dL) (APACHE II Score: 24). The severity of the clinical picture induced us to additionally apply blood purification therapy with CPFA. The procedure was performed for 6–8 h (daily, for three consecutive days) with a four-pump blood-purification machine (LYNDA®; Bellco, Mirandola, Italy) and a kit (ABL814; Bellco SpA) including a polyethersulfone plasmafilter (0.5 m2), an adsorbent cartridge containing styrene resin with macroporous structure (50 000 m2/cartridge), and a highly permeable polyethersulfone haemofilter (1.4 m2) where convective exchanges may be applied to the reconstituted blood in a post-dilutional mode. Blood flow rate was set to 180 mL/min, plasma separation rate was adjusted to 15% in the plasmafilter and haemofiltration was performed at the ultrafiltration rate of 25–30 mL/kg/h, aiming to a weight loss of 1 kg per session. Pro-inflammatory cytokines [interleukin (IL)-6, tumour necrosis factor (TNF)-α, interferon-α, monocyte chemotactic protein (MCP)-1], pro-calcitonin (PCT) and C-reactive protein (CRP) were measured before every CPFA session and after the last one. Clinical conditions rapidly improved. Twenty-four hours after the first CPFA session CPAP was withdrawn. The following day, the patient was not confused and converted back into sinus rhythm. After 4 days, the oxygen saturation was 97% while breathing in room air. During the 3 days in which CPFA treatments were carried out, serum levels of pro-inflammatory cytokines, PCT and CRP decreased progressively (Figure 2A–E). APACHE II progressively decreased, reaching a score of 9 five days after the first CPFA session (Figure 2F). A control chest HRCT was performed after 9 days from the admission, confirming a substantial improvement of the acute respiratory disease (Figure 1B).
Table 1.
Patient’s main clinical and laboratory data registered at the admission, before each CPFA session and 48 h after the last one
| Parameters | Admission | 1st CPFA session | 2nd CPFA session | 3rd CPFA session | 48 h after CPFA cycle |
| Serum creatinine (mg/dL) | 7.5 | 8 | 7.7 | 7.0 | 6.2 |
| Urea (mg/dL) | 143 | 170 | 141 | 147 | 136 |
| White blood cells (cells/mm3) | 7,240 | 7,360 | 6,200 | 6,090 | 6,390 |
| Lymphocytes (%) | 1.3 | 3.3 | 6.5 | 6.7 | 9.5 |
| Mean arterial pressure (mmHg) | 95 | 120 | 103 | 108 | 102 |
| Temperature (°C) | 37.8 | 38 | 37.1 | 36.5 | 36.0 |
| Arterial pO2 (mmHg) | 48.7a | 49.8b | 84.3b | 90.3c | 93.1a |
| Arterial oxygen saturation (%) | 86.7a | 84.2b | 97.1b | 96.0c | 98.0a |
| Serum bicarbonates (mmol/L) | 23.3 | 20.8 | 23.7 | 23.8 | 24.0 |
| Lactic dehydrogenase (IU/L) | 1001 | 1114 | 983 | 958 | 817 |
| Aspartate aminotransferase (IU/L) | 44 | 93 | 54 | 37 | 30 |
| Alanine aminotransferase (IU/L) | 33 | 91 | 71 | 53 | 43 |
While breathing room air.
Venturi Mask (FiO2 = 0.4).
Venturi mask (FiO2 = 0.35).
Fig. 1.
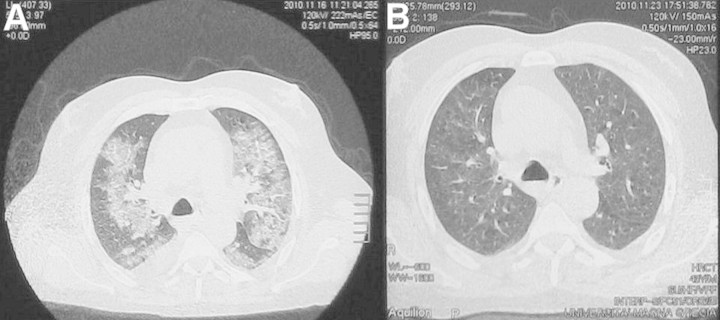
HRCT scan of the chest before (A) and after (B) CPFA treatments.
Fig. 2.
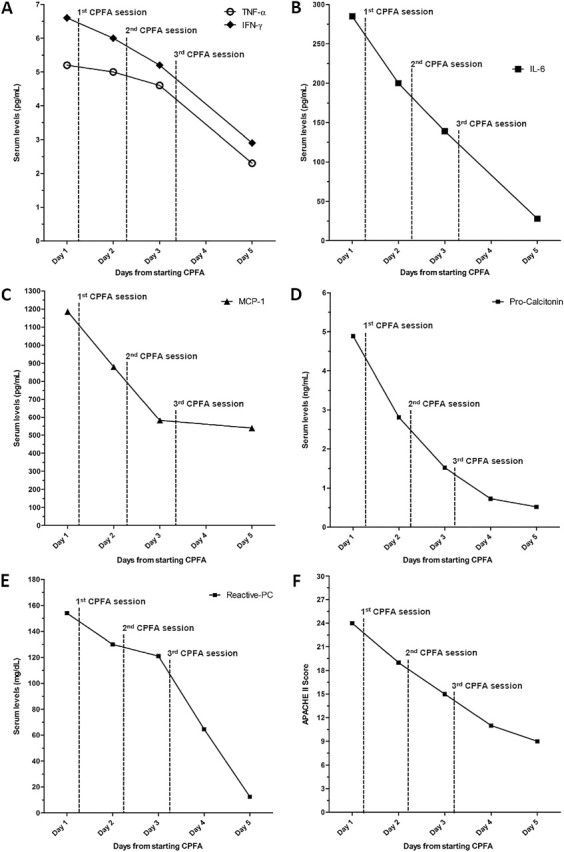
Trend of APACHE II score and of cytokines, PCT and CRP levels from admission.
Discussion
CPFA has been proposed as an emerging treatment aimed at restoring immune function in patients with sepsis-related multiorgan dysfunction by non-selectively reducing the circulating levels of inflammatory mediators [4, 5]. However, evidence-based data about the clinical efficacy of this technique are still lacking [6, 7] not only in the course of sepsis but even in less acute inflammatory conditions that do not fulfill the traditional criteria for sepsis. We report the case of a critically ill patient with ARDS and AKI in which CPFA was successfully used as part of the treatment.
ARDS is a severe disease characterized by diffuse inflammation of lung parenchyma which determines a large release of cytokines and other pro-inflammatory mediators secreted by local endothelial and epithelial cells, such as TNF-α, IL-6 and MCP-1 [8], that in turn determine the release of acute-phase reactants (mainly CRP and PCT). In particular, it has been reported that high plasmatic levels of IL-6 are associated with increased morbidity and mortality in patients with acute lung injury [9]. If ARDS is not promptly treated, the amount of inflammatory mediators released by the lungs may result in systemic inflammatory response syndrome or sepsis. This was not the case for our patient. In fact, he developed ARDS and an acute deterioration of renal function but was immediately treated with antimicrobial agents and CPFA. The decision of employing CPFA as renal replacement technique was taken because the patient showed fluid overload and appeared at high risk of sepsis. In the days in which CPFA was performed, we observed a progressive reduction of serum pro-inflammatory cytokines, in particular IL-6 and TNF-α (Figure 2A and B). Such decrease was associated with a parallel decline in the serum levels of CRP and PCT (and thus of the systemic inflammatory state) (Figure 2D and E) and with a rapid improvement of the clinical conditions and a decrease of the APACHE II score (Figure 2F). It is difficult to establish if CPFA played a relevant role in this case. However, it is well known that the mortality in patients with ARDS is very high [10] and that the therapeutic measures, which attenuate the inflammatory state and maintain a slightly negative fluid balance, can improve the outcome in these patients [11, 12]. Steroids are part of the therapy, but our patient was already on steroid treatment before developing pneumonia and this therapy was maintained throughout the course of the acute disease so that the fall in plasma cytokines that we observed after CPFA was started cannot be entirely ascribed to steroids. Of course, we cannot exclude that the observed changes in cytokine levels reflect merely an antibiotic-related improvement of the disease, but the rapidity of the cytokine decline and the temporal association with CPFA therapy strongly suggest a beneficial role for this procedure in our patients.
In conclusion, CPFA may have played a role in the favourable outcome of our patient with AKI and ARDS by reducing inflammation and fluid overload. This case report also suggests that starting CPFA early as part of the treatment of severe acute inflammatory diseases could help improve the survival of critically ill patients, possibly preventing the onset of sepsis, although this hypothesis needs to be verified in specifically designed trials.
Acknowledgments
Funding. There was no financial support for this study.
Conflict of interest statement. None declared.
References
- 1.Bellomo R, Tetta C., Ronco C. Coupled plasma filtration adsorption. Intensive Care Med. 2003;29:1222–1228. doi: 10.1007/s00134-003-1796-x. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, Brendolan A, D'Intini V, et al. Coupled plasma filtration adsorption: rationale, technical development and early clinical experience. Blood Purif. 2003;21:409–416. doi: 10.1159/000073444. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, Brendolan A, Lonnemann G, et al. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med. 2002;30:1250–1255. doi: 10.1097/00003246-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Formica M, Inguaggiato P, Bainotti S, et al. Coupled plasma filtration adsorption. Contrib Nephrol. 2007;156:405–410. doi: 10.1159/000102131. [DOI] [PubMed] [Google Scholar]
- 5.Mao HJ, Yu S, Yu XB, et al. Effects of coupled plasma filtration adsorption on immune function of patients with multiple organ dysfunction syndrome. Int J Artif Organs. 2009;32:31–38. doi: 10.1177/039139880903200104. [DOI] [PubMed] [Google Scholar]
- 6.Page M, Rimmele T. [Coupled plasma filtration adsorption: rationale and perspectives in septic shock] Can J Anaesth. 2008;55:847–852. doi: 10.1007/BF03034056. [DOI] [PubMed] [Google Scholar]
- 7.Stengl M, Sykora R, Chvojka J, et al. Differential effects of hemofiltration and of coupled plasma filtration adsorption on cardiac repolarization in pigs with hyperdynamic septic shock. Shock. 2010;33:101–115. doi: 10.1097/SHK.0b013e3181ab6359. [DOI] [PubMed] [Google Scholar]
- 8.Martin TR. Lung cytokines and ARDS: Roger S. Mitchell Lecture. Chest. 1999;116(1 Suppl):2S–8S. doi: 10.1378/chest.116.suppl_1.2s. [DOI] [PubMed] [Google Scholar]
- 9.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. ; discussion 230-232. [DOI] [PubMed] [Google Scholar]
- 10.MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care. 2005;11:43–49. doi: 10.1097/00075198-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg AL, Dechert RE, Park PK, et al. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 12.Meduri GU, Marik PE, Chrousos GP, et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34:61–69. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]


