Abstract
We report a case of acute renal failure (ARF) and bilateral nephromegaly in a patient with a history of Crohn’s disease and treatment with azathioprine. Kidney biopsy revealed diffuse renal infiltration by precursor T-cell lymphoblastic lymphoma (T-LBL). At the time of diagnosis, no extrarenal manifestations of the lymphoma were detectable and therefore the lymphoma was categorized as primary renal lymphoma (PRL). Thus far, precursor T-LBL presenting as PRL has not been described before. We emphasize that in patients with ARF and bilateral renal enlargement, renal lymphoma is an important differential diagnostic consideration.
Keywords: acute renal failure, bilateral nephromegaly, precursor T-lymphoblastic lymphoma, (primary) renal lymphoma
Background
Renal involvement of systemic lymphoma is a frequent feature with a prevalence of up to 50% in autopsy studies [1]. Primary involvement of the kidney, however, is very rare (1%) [2]. We report a case of precursor T-cell lymphoblastic lymphoma (T-LBL) presenting as primary renal lymphoma (PRL) with acute renal failure (ARF) and bilateral nephromegaly in a patient with history of Crohn’s disease and prior treatment with azathioprine.
Case report
A 23-year-old man presented at the emergency department with ARF. The patient had a medical history of Crohn’s disease for which he had been treated with prednisone and azathioprine until 2006, followed by a laparoscopic ileocaecal resection. Four months before presentation, he was admitted elsewhere because of chest pain, for which acetylcystein and esomeprazole were started. At that time, serum creatinine levels were within normal limits. At the current consultation, serum creatinine level was 548 μmol/L. He experienced chest pain which was continuously present during the day, and not related to exercise. No radiation of the pain nor dyspnoea were present. In the past 4 months, he had lost 3 kg of body weight. Physical examination revealed a blood pressure of 150/80 mmHg, but otherwise normal vital signs. Except for an erythema on the malar eminences, no abnormalities were noted, especially no oedema, no pericardial friction rub, no lymphadenopathy, nor hepatosplenomegaly. Further laboratory values showed haemoglobin 7.9 mmol/L, white blood cell count 13.4 × 109/L, 68% neutrophils, trombocytes 330 × 109/L, 4.4 g/L and C-reactive protein 45.5 mg/L. Serology of human immunodeficiency virus, hepatitis B virus (HBV) and hepatitis C virus (HCV) was negative. Moreover, normal C3 and C4 and negative results of anti-double strain DNA, anti-nuclear antibody, anti-nuclear cytoplasmatic antibody and anti-GBM antibody were found. Urinalysis showed microscopic haematuria (3+) and proteinuria (1+), and urine microscopic examination showed >20 red blood cells/high-power field but no red blood cell casts. A 24-h urine collection measurement showed 230 mg of protein. Renal ultrasound showed bilateral nephromegaly of 15, respectively, 14.6 cm in length without evidence of obstruction. At this stage, a kidney biopsy was performed which showed interstitial infiltration of medium-sized atypical lymphoid cells by light microscopy (Figure 1). Immunohistochemical stains of these atypical lymphocytes were strongly positive for CD3, TdT, Ki67 and CD99 and negative for CD20 and Epstein–Barr virus (EBV). The morphology and the immunohistochemical marker profile were consistent with the WHO classification of precursor T-lymphoblastic leukaemia/lymphoma. Additional molecular diagnostic analysis supported the diagnosis of malignant T-lineage lymphoma. The tubuli and glomeruli did not show apparent histopathological abnormalities. Total body computerized tomography, a bone marrow biopsy and a spinal fluid aspiration were performed to stage the lymphoma. Apart from diffusely enlarged kidneys, these investigations were negative: neither lymphadenopathy nor pericardial effusion was seen. Based on these results, the lymphoma was classified as PRL and the patient started immediately with remission-induction chemotherapy receiving prednisolone, cyclophosphamide, daunorubicin, vincristin, L-asparaginase and intrathecal methotrexate for induction. After completion of the consolidation phase, kidney function improved to a serum creatinine level of 74 μmol/L. Recently, the patient finished his almost 2 years of maintenance treatment. At re-evaluation, no evidence of renal or extrarenal lymphoma was present and kidney function remained normal.
Fig. 1.
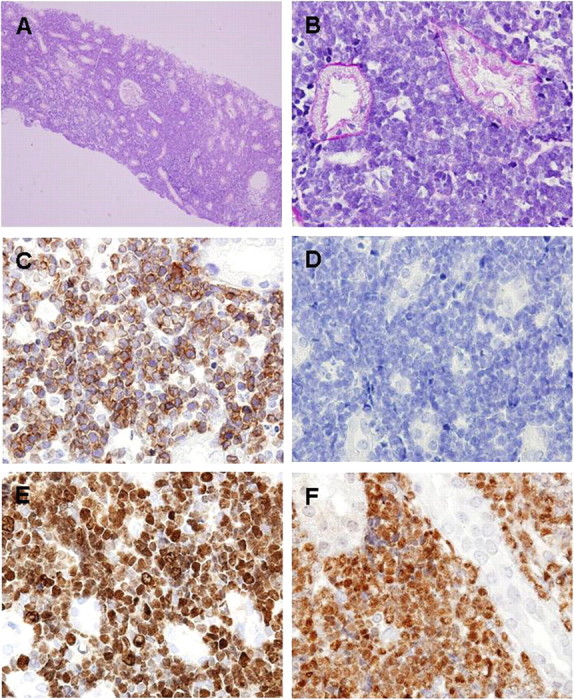
Findings in the renal biopsy specimen. A diffuse interstitial infiltration of medium-sized atypical lymphoid blasts with condensed chromatin without evident nucleoli, resulting in constricted tubules, was observed in light microscopy. Glomeruli and tubuli did not show apparent histopathological abnormalities (A and B). Immunohistochemical stains of these atypical lymphocytes were strongly positive for (cytoplasmic) CD3 (C), negative for CD20 (D) and nuclei positive for TdT (E) and Ki67 (F).
Discussion
Most reported cases of PRL are of B-cell lineage and only a few case reports on T-cell PRL have been published [3,4]. To our knowledge, a precursor T-LBL presenting as a PRL has not been described before. A precursor T-LBL is a highly aggressive neoplasm of T-cell lineage, arising from precursor T lymphoblasts. Precursor T-LBL is differentiated from T-cell acute lymphoblastic leukaemia by bone marrow biopsy or aspiration, and the diagnosis precursor T-LBL is established when <25% blasts are present. In our patient, no blasts were present in the bone marrow.
Since the kidneys do not contain lymphoid tissue, controversy exists whether PRL should be considered as a distinct disease entity. Some have postulated that lymphoma can arise from potential lymphoid tissue in the renal hilum [4] or capsule [5]. Others suggest chronic inflammation as an initiator for lymphoma [6]. For this reason, the diagnosis of PRL should only be made in the presence of a biopsy-proven lymphomatous renal infiltration, a non-obstructive nephromegaly, and in the absence of extrarenal lymphoma [7]. Furthermore, some authors also stipulate improvement of renal failure after chemotherapy [8].
Lymphoma may cause ARF by multiple mechanisms. A direct effect may result from ureteral obstruction, the compression of renal arteries, veins and tubules and/or bilateral parenchymal infiltration, as seen in the present case. PRL may indirectly lead to ARF by causing hypercalcaemia and/or haemolysis. Treatment-related effects include tumour lysis syndrome, nephrotoxicity of chemotherapeutic agents and radiation nephritis.
A role of immunosuppressive agents in the development of lymphoproliferative disease, predominantly EBV-associated B-cell lymphomas, has been known for many years. Of note, we describe a case of T-cell lymphoma without an EBV association. A meta-analysis by Kandiel et al. revealed a 4-fold increased risk of lymphoma in patients with inflammatory bowel disease, treated with azathioprine [9]. However, independently of the use of immunosuppressive agents, patients with Crohn’s disease itself seem to have no substantially increased risk to develop a malignant lymphoma [10].
The prognosis of primary renal T-cell lymphoma is difficult to estimate as PRL only represents a minor fraction of the total number of primary extranodal lymphomas. Only two other cases with ARF have been described in the literature. One patient died within 13 months after initial presentation [4] the other was disease-free 17 months post-diagnosis [3]. In general, adult patients with precursor T-LBL have a poor prognosis, despite the significant progress that has been made with the introduction of more aggressive treatment protocols.
In conclusion, we report a case of primary renal T-cell lymphoma presenting with ARF and bilateral nephromegaly in a patient with a history of Crohn’s disease and treatment with azathioprine. Renal biopsy was diagnostic of diffuse precursor T-LBL. In patients with ARF and bilateral renal enlargement, renal lymphoma is an important differential diagnostic consideration.
Acknowledgments
Disclosures. No conflicts of interests to declare. No author received any financial compensation for collaboration on this article.
References
- 1.Urban BA, Fishman EK. Renal lymphoma: CT patterns with emphasis on helical CT. Radiographics. 2000;20:197–212. doi: 10.1148/radiographics.20.1.g00ja09197. [DOI] [PubMed] [Google Scholar]
- 2.Barreto F, Dall'Oglio MF, Srougi M. Renal lymphoma. Atypical presentation of a renal tumor. Int Braz J Urol. 2006;32:190–192. doi: 10.1590/s1677-55382006000200010. [DOI] [PubMed] [Google Scholar]
- 3.Miyake JS, Fitterer S, Houghton DC. Diagnosis and characterization of non-Hodgkin's lymphoma in a patient with acute renal failure. Am J Kidney Dis. 1990;16:262–263. doi: 10.1016/s0272-6386(12)81028-6. [DOI] [PubMed] [Google Scholar]
- 4.O'Riordan E, Reeve R, Houghton JB, et al. Primary bilateral T-cell renal lymphoma presenting with sudden loss of renal function. Nephrol Dial Transplant. 2001;16:1487–1489. doi: 10.1093/ndt/16.7.1487. [DOI] [PubMed] [Google Scholar]
- 5.Salem Y, Pagliaro LC, Manyak MJ. Primary small noncleaved cell lymphoma of kidney. Urology. 1993;42:331–335. doi: 10.1016/0090-4295(93)90627-m. [DOI] [PubMed] [Google Scholar]
- 6.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 7.Da'as N, Polliack A, Cohen Y, et al. Kidney involvement and renal manifestations in non-Hodgkin's lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol. 2001;67:158–164. doi: 10.1034/j.1600-0609.2001.5790493.x. [DOI] [PubMed] [Google Scholar]
- 8.Malbrain ML, Lambrecht GL, Daelemans R, et al. Acute renal failure due to bilateral lymphomatous infiltrates. Primary extranodal non-Hodgkin's lymphoma (p-EN-NHL) of the kidneys: does it really exist? Clin Nephrol. 1994;42:163–169. [PubMed] [Google Scholar]
- 9.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JD, Bilker WB, Brensinger C, et al. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology. 2001;121:1080–1087. doi: 10.1053/gast.2001.28703. [DOI] [PubMed] [Google Scholar]


