Abstract
Background
Exposure to arsenic is one of the major global health problems, affecting > 300 million people worldwide, but arsenic’s effects on human reproduction are uncertain.
Objectives
We conducted a systematic review and meta-analysis to examine the association between arsenic and adverse pregnancy outcomes/infant mortality.
Methods
We searched PubMed and Ovid MEDLINE (from 1946 through July 2013) and EMBASE (from 1988 through July 2013) databases and the reference lists of reviews and relevant articles. Studies satisfying our a priori eligibility criteria were evaluated independently by two authors.
Results
Our systematic search yielded 888 articles; of these, 23 were included in the systematic review. Sixteen provided sufficient data for our quantitative analysis. Arsenic in groundwater (≥ 50 μg/L) was associated with increased risk of spontaneous abortion (6 studies: OR = 1.98; 95% CI: 1.27, 3.10), stillbirth (9 studies: OR = 1.77; 95% CI: 1.32, 2.36), moderate risk of neonatal mortality (5 studies: OR = 1.51; 95% CI: 1.28, 1.78), and infant mortality (7 studies: OR = 1.35; 95% CI: 1.12, 1.62). Exposure to environmental arsenic was associated with a significant reduction in birth weight (4 studies: β = –53.2 g; 95% CI: –94.9, –11.4). There was paucity of evidence for low-to-moderate arsenic dose.
Conclusions
Arsenic is associated with adverse pregnancy outcomes and infant mortality. The interpretation of the causal association is hampered by methodological challenges and limited number of studies on dose response. Exposure to arsenic continues to be a major global health issue, and we therefore advocate for high-quality prospective studies that include individual-level data to quantify the impact of arsenic on adverse pregnancy outcomes/infant mortality.
Citation
Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K, Cobbina SJ, Nketiah-Amponsah E, Namujju PB, Obiri S, Dzodzomenyo M. 2015. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect 123:412–421; http://dx.doi.org/10.1289/ehp.1307894
Introduction
Arsenic contamination of drinking water, air, food, and beverages is one of the major global health problems (Essumang 2009; Essumang et al. 2007; Hughes 2006; Navas-Acien and Nachman 2013; Obiri et al. 2010) that affect > 300 million people worldwide. This includes an estimated 13 million people in the United States and about 70 million people in Bangladesh (Murcott 2012). At concentrations > 50 μg/L, inorganic arsenic (iAs) has been associated with elevated risk of cancer (e.g., bladder, kidney, liver, lung, skin, prostate) (Ahamed et al. 2006b; McDonald et al. 2007; Mink et al. 2008; Steinmaus et al. 2000, 2003; Walvekar et al. 2007), cardiovascular diseases (Moon et al. 2013; Navas-Acien et al. 2005), high blood pressure (Abhyankar et al. 2012; Moon et al. 2013; Navas-Acien et al. 2005), anemia in pregnancy (Hopenhayn et al. 2006; Navas-Acien et al. 2006), mortality from respiratory diseases in both adults and children (Ahamed et al. 2006a; Ferreccio and Sancha 2006; Walvekar et al. 2007), diabetes in adults (Navas-Acien et al. 2006), and neurodevelopment problems (Hamadani et al. 2011). At concentrations around 10 μg/L, which is considered safe by the World Health Organization’s (WHO) provisional guideline (WHO 2011), iAs may still cause cancer in approximately 0.1–0.3% and increased systolic blood pressure in women 6 weeks postpartum (International Agency for Research on Cancer 2004; Kwok 2007). iAs easily crosses human and animal placenta and has been demonstrated to increase the risk of impaired fetal growth and infant mortality in laboratory animal studies (Navarro et al. 2004; Smith and Steinmaus 2009; Vahter 2009). Several epidemiologic studies (e.g., Cherry et al. 2010; Myers et al. 2010; Rahman et al. 2010) have examined the relation between arsenic and adverse pregnancy outcomes/infant mortality, and the findings are equivocal. Our understanding of arsenic exposure and adverse pregnancy outcomes is limited and, at best, fragmented. To our knowledge, no systematic review and/or meta-analysis has reported on the effect of arsenic on human pregnancy and infant health. Given the widespread low, moderate, and high arsenic exposure in the general population, an understanding of the impact of iAs on maternal and fetal health is relevant for public health policy.
To fill this gap, we conducted a systematic review and meta-analysis of epidemiologic studies to examine the association between arsenic exposure and the risk of spontaneous abortion, stillbirth, preterm delivery, birth weight, and neonatal/infant mortality.
Methods
Search strategy and study selection. This study was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) group (Moher et al. 2009). We searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Ovid MEDLINE (http://ovidsp.tx.ovid.com) (from 1946 through July 2013) and EMBASE (http://www.embase.com/login) (from 1988 through July 2013) databases (Figure 1), using the terms “arsenic,” “arsenicals,” “arsenite,” “arsenate” and “abortion, spontaneous,” “fetal mortality,” “preterm delivery,” “low birthweight,” “birthweight,” “infant mortality,” “neonatal mortality” (see Supplemental Material, “Search Strategy”). In addition, we searched the reference lists of reviews (Bloom et al. 2010; Smith and Steinmaus 2009; Vahter 2009) and potentially relevant articles. Two authors (R.Q. and F.A.A.) independently evaluated the articles. Studies that fulfilled the following a priori eligibility criteria were included if the study a) was an original study; b) was a cross-sectional, or a case–control or a cohort design; c) reported on any one or more of the following outcomes: spontaneous abortion, stillbirth, preterm delivery, birth weight, and neonatal/infant mortality; and d) presented data on arsenic exposure determined using environmental measures (arsenic in drinking water or airborne arsenic, or arsenic in soil), biomarkers, or indirect measures (e.g., residing in arsenic endemic area). Our exclusion criteria were a) a study was an experimental or a case report or a case series or a letter, b) a study was of arsenic compounds for which human exposure was unlikely [e.g., arsenic in roots of plants (Landgren 1996)], c) a study used job title or living close to a smelter house as surrogate for arsenic exposure, and d) a study did not include our relationships of interest.
Figure 1.
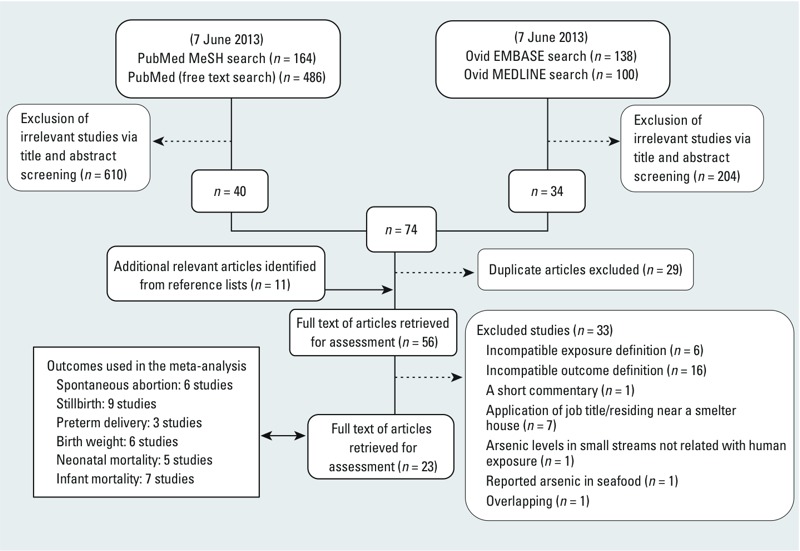
Study selection flow diagram.
If more than one report was published from the same study, the most recent study or the study using the best assessment of arsenic and/or outcome was included. For studies that reported estimates for more than one biomarker, the estimate for the most appropriate biomarker was preferred. The order of preference was as follows: nail > hair > urine. If a study provided estimates for water and a biomarker, the estimate from the latter was used.
Data extraction and quality assessment. Most relevant characteristics of eligible studies including study design, study size, location and country of study, method of arsenic assessment, exposure marker for arsenic, exposure contrast, exposure dose, type of adverse pregnancy/infant mortality and their definitions, year of publication, year of data collection, adjustment for adequate confounders, and study results (i.e., measures of association) were recorded in a standard data extraction form (Quansah and Jaakkola 2010) independently by two authors (R.Q. and F.A.A.). Any discrepancies were resolved by consensus. R.Q. and F.A.A. applied the Newcastle–Ottawa Scale (Wells et al. 2009) for observational studies to assess quality of eligible studies, with the maximum score of 9. Studies scoring ≥ 7 were categorized as high quality (see Supplemental Material, Tables S1 and S2).
Statistical methods. First, odds ratio (OR) or relative risk (RR) and their 95% confidence intervals (CIs) were derived or abstracted from eligible studies. Almost all the studies presented ORs and their 95% CIs; therefore, we used ORs in our analysis. One study (Ahmad et al. 2001) presented adjusted ORs and exact p-values but not the 95% CI, so we calculated the 95% CI from the p-values following Borenstein et al. (2009). Two studies (Guan et al. 2012; Rahman et al. 2007) presented RRs, and these were converted to ORs (Zhang and Yu 1998). Some relevant studies presented ORs for more than two exposure levels for the outcomes; therefore, in the meta-analysis we calculated summary ORs comparing our outcomes of interest (adverse pregnancy outcomes/infant mortality) in the highest (exposed group) and lowest (reference group) arsenic exposure categories presented in the studies. The exposed groups were heterogeneous and consisted of populations exposed to arsenic dose above the WHO guideline (i.e., > 10 μg/L) (WHO 2011). We separated the groups into high dose (exposed to ≥ 50 μg/L) and low-to-moderate dose (exposed to < 50 μg/L) for further analysis (explained below).
We applied the random-effects model (Borenstein et al. 2009) because we anticipated heterogeneity in the study-specific estimates. In the forest plots, we presented summary ORs of the random-effects model. Heterogeneity was computed using the Q (p < 0.1 considered significant), and I2-statistics (I2-statistic > 50% indicates high, 25–50% moderate, and < 25% low heterogeneity). We examined the influence of various characteristics on the study-specific effect estimates by stratifying the analysis by a) arsenic dose [i.e., high arsenic dose (> 50 μg/L) vs. low-to-moderate arsenic dose (< 50 μg/L)] and b) arsenic measured using individual data versus group data. We also performed a series of sensitivity analyses. First, we investigated the relative influence of each study on the summary OR by omitting each study one at a time. None of the studies had substantial influence on the summary ORs for our relations of interest and this finding was not reported. Second, we restricted the analysis to high-quality studies, prospective cohort studies, and studies adjusting for potential adequate confounders (see Supplemental Material, Table S3) documented in the literature (e.g., Di Mario et al. 2007; Ghosh 2012; Kramer 1987, 2003; McClure et al. 2006; Moss et al. 2002; Shah et al. 2011). We also presented dose response for studies with at least three exposure levels graphically. Publication bias was explored with funnel plots. The trim and fill method was used to assess the potential impact of missing studies in the funnel plot. Statistical analysis was performed using STATA software version 9 (StataCorp, College Station, TX, USA).
Results
Study characteristics. Our systematic literature search strategy is shown in Figure 1. A total of 888 studies were retrieved, from which 56 studies were reviewed in depth. Twenty-three studies fulfilled our a priori inclusion criteria (Table 1) for this systematic review. Data from 16 studies were included in our quantitative analysis (see Supplemental Material, Tables S4 and S5). Thirty-three studies were excluded for various reasons (see Supplemental Material, Table S6). We had no data from the authors of two studies on birth weight (Fei et al. 2013; Guan et al. 2012) to calculate 95% CIs, so we included the studies only in our qualitative analysis. Five studies (Ahamed et al. 2006b; Chakraborti et al. 2003; Mukherjee et al. 2005; Rahman et al. 2005; Sen and Chaudhuri 2008) did not control for potential confounders, and they were also included in our qualitative analysis. Five studies (Cherry et al. 2008, 2010; Hopenhayn-Rich et al. 2000; Milton et al. 2005; Myers et al. 2010) were ecological retrospective cohort designs. Two studies were ecological case–control designs (Aschengrau et al. 1989; Ihrig et al. 1998). Only 1 (Rahman et al. 2007) of the 5 prospective cohort designs was an ecological study. Seven of the 10 cross-sectional designs were ecological studies. Nine studies were conducted in Bangladesh, 5 in India, 3 in China, 2 in Chile, 1 in Taiwan, and 3 in the United States. Twenty-two studies were conducted in populations exposed to arsenic in drinking water. Of these, 6 applied biomarkers including urine, maternal/cord/placenta blood, hair, and nail; and the remaining studies measured arsenic dose at the region/village/household level. Ihrig et al. (1998) measured arsenic dose in airborne emissions. Huyck et al. (2007) measured maternal hair arsenic dose at first prenatal visit, maternal hair arsenic dose at birth, and maternal nail arsenic dose at first prenatal visit, but the estimates of the latter biomarker was considered suitable and included in the meta-analysis. There were 10 reports on spontaneous abortion, 14 on stillbirth, 3 on preterm delivery, 6 on birth weight, 5 on neonatal mortality, and 7 on infant mortality. Eligible studies applied either questionnaire/interview or hospital/medical records or national registers to ascertain information on the outcomes of interest. Most of the studies (Table 1) scored low on the Newcastle–Ottawa Scale for several reasons, including bias associated with selection of study population, measurement of arsenic exposure, lack of individual arsenic data, inappropriate definition of cases/controls, inappropriate comparable reference, and a lack of adequate adjustment for potential confounders (Table 1).
Table 1.
Characteristics of studies included in the systematic review and meta-analysis.
| Sources (study design) | Location | Study population | Arsenic concentration | Outcome studied | Confounders adjusted for | Total score on NOS | ||
|---|---|---|---|---|---|---|---|---|
| Marker for exposure | Exposure contrast | Range, median, or mean | ||||||
| Fei et al. 2013a,b (PCO) | New Hamsphire (USA) | 133 pregnant women | Arsenic levels in urine | NA | Not reported | Birth weight | Infant sex, maternal age, gestational age | 7/9 |
| Guan et al. 2012b,c (CS) | Dalian (China) | 125 mother–infant pairs | Arsenic levels in maternal and cord blood | Arsenic-affected area (590 μg/L) vs. arsenic-free area | Not reported | Birth weight | Maternal age, BMI, parity, gestational age at delivery, maternal education, maternal secondhand smoke exposure, infant sex | 5/9 |
| Cherry et al. 2010c,d (RCO) | Gonoshasthaya Kendra villages (Bangladesh) | 934 infant mortality occurring in designated area, 2001–2003 | Arsenic levels in tube-well water | ≥ 50 μg/L vs. < 10 μg/L | 0.05–166 μg/L | Infant mortality | First pregnancies, others with no formal education, mothers designated as destitute | 7/9 |
| Myers et al. 2010c,d (RCO) | Bayingnormen (Mongolia, China) | 9,890 singleton deliveries of mothers | Arsenic levels in tube-well water | > 50 μg/L vs. ≤ 50 μg/L | UD–1,200 μg/L | Birth weight, preterm delivery, stillbirth, and neonatal mortality | Maternal age, gravidity, infant sex for the analysis of birth weight and maternal age, gravidity, infant sex adequacy for the analysis of preterm delivery, stillbirth, and neonatal mortality | 7/9 |
| Rahman et al. 2010b,c (PCO) | Matlab district (Bangladesh) | 2,924 pregnant women | Arsenic levels in urine | 249–1,253 μg/L vs. < 33 μg/L (spontaneous abortion)268–2,019 μg/L vs. < 38 μg/L (stillbirth)268–2,019 vs. < 38 μg/L (infant mortality) | UD–1,253 μg/L | Spontaneous abortion, stillbirth, infant mortality | No significant confounder was found | 7/9 |
| Rahman et al. 2009b,c (PCO) | Matlab (Bangladesh) | 1,578 women with single births | Arsenic concentrations in urine | ≥ 100 μg/L vs. < 100 μg/L | 6–978 μg/L | Birth weight | Asset score, BMI, height, age, education, season, gestational age at birth, sex of infant | 8/9 |
| Cherry et al. 2008c,d (RCO) | Villages in 13 subdistricts (Bangladesh) | 30,984 pregnancies and outcomes | Average arsenic concentrations in hand-pumped well water | ≥ 50 μg/L vs. < 0.10 μg/L | UD–81 μg/L | Stillbirth | Age, sex, previous pregnancy, previous stillbirth, low socioeconomic status, maternal education, paternal education, maternal smoking, mother high BP, mother edema, gestational age, birth weight, home delivery | 8/9 |
| Sen and Chaudhuri 2008c,d,e (CS) | Villages located in North 24 Parganas district (states of West Bengal) | Pregnancy outcomes of 240 married women | Arsenic levels in tube-well water | 600 μg/L vs. < 10 μg/L | 10–600 μg/L | Spontaneous abortion and stillbirth | None | 2/9 |
| Huyck et al. 2007b,c (PCO) | 42 villages in Sirajdikhan Upakila of Munshigani district (Bangladesh) | 49 women ≥ 18 years of age | Arsenic levels in maternal hair at first visit | ≥ 2.70 μg/g vs. < 0.28 μg/g | 0.14–3.28 μg/g | Birth weight | Gestational age at first prenatal visit, maternal weight gain, birth gestational age, and activity level during pregnancy | 7/9 |
| Rahman et al. 2007c,d (PCO) | Matlab (Bangladesh) | 29,134 pregnancies identified by the HDSS in 1991–2000 | Arsenic levels in tube-well water | ≥ 409 μg/L vs. < 10 μg/L | Median, 224 μg/L | Fetal loss, infant mortality, neonatal | Age, parity, education, and socioeconomic status | 7/9 |
| Ahamed et al. 2006a, 2006bc,d,e (CS) | Eruani village (Bangladesh) | 56 pregnancy outcomes in women of reproductive age | Arsenic levels in tube-well water | Exposed area (501–1,200 μg/L) vs. control area | 501–1,200 μg/L | Spontaneous abortion and stillbirth | None | 1/9 |
| von Ehrenstein et al. 2006c,d (CS) | 21 villages in West Bengali (south 24-Parganas district) (India) | 202 married women, 20–40 years of age | Arsenic levels in tube-well water | ≥ 200 μg/L vs. < 50 μg/L | Mean = 101.7 μg/L | Spontaneous abortion, stillbirth, neonatal mortality, infant mortality | Mother’s age at child’s birth, BMI, maternal education, education of the head of the household, and type of housing material | 3/9 |
| Milton et al. 2005c,d (CS) | 29 villages in Comilla district, 2 villages in the Chandpur district, 43 villages in the Chaudanga district (Bangladesh) | 533 ever-married women, 15–49 years of age | Arsenic levels in tube-well water | > 50 μg/L vs. ≤ 50 μg/L | UD–1,710 μg/L | Spontaneous abortion, stillbirth, and neonatal mortality | Height, history of hypertension and diabetes, and age at first pregnancy for neonatal mortality | 3/9 |
| Mukherjee et al. 2005c,d,e (CS) | Murshidabad (West Bengal, India) | 17 married women of reproductive age (18–40 years) with at least 1 pregnancy | Arsenic levels in drinking water | Exposed area (401–1,474 μg/L) vs. nonexposed area (< 3 μg/L) | 401–1,474 μg/L | Spontaneous abortion and stillbirth | None | 1/9 |
| Rahman et al. 2005c,d,e (CS) | Jalangi block (India) | 13 married women of reproductive age (18–40 years) | Arsenic levels in drinking water | Women in exposed areas (501–1,474 μg/L) vs. women in control area (< 10 μg/L) | Not reported | Spontaneous abortion and stillbirth | None | 1/9 |
| Chakraborti et al. 2003c,d,e (CS) | Semria Ojha Patti village of Ara (Bhoipur, India) | 16 women | Arsenic levels in tubes-well water | 463–1,025 μg/L vs. 7–39 μg/L | 7–1,025 μg/L | Stillbirth | None | 1/9 |
| Guo et al. 2003a,d (CS) | Villages in Wuyan County (Inner Mongolia, China) | 224 women | Arsenic levels in well water | Exposed area (43 μg/L) vs. nonexposed area (9.6 μg/L) | Not reported | Spontaneous abortion | Sex, age, smoking and alcohol consumption | 3/9 |
| Hopenhayn et al. 2003a,d (PCO) | Antofagasta and Valparaiso (Chile) | 844 singleton mothers 18–45 years of age | Arsenic levels in water | 40 μg/L vs. < 1 μg/L | 32.9–52.7 μg/L | Birth weight | Location, calendar time, arsenic exposure | 6/9 |
| Yang et al. 2003a,d (RCO) | 18 villages in 4 township in Lanyang Basin (Taiwan) | 18,259 singleton births | High arsenic–exposed community used as a surrogate | Exposed area (UD–3,590 μg/L) vs. nonexposed area | UD–3.59 ppm | Preterm delivery, birth weight | Maternal age, marital status, maternal education, infant sex | 6/9 |
| Ahmad et al. 2001c,d (CS) | Village of Samta in thana Sharsha, Jessore district; village of Katiarchar in Sadar thana, Kishorgonj district (Bangladesh) | 192 married women of reproductive age (15–49 years) | Arsenic levels in tube-well water | > 50 μg/L vs. ≤ 0.2 μg/L | 200–450 μg/L | Spontaneous abortion, stillbirth, and preterm birth | Socioeconomic status, education, and age at marriage | 3/9 |
| Hopenhayn-Rich et al. 2000c,d (RCO) | Antofagasta and Valparaiso (Chile) | Mortality of infants, 1950–1996 | Arsenic levels in public water | > 50 vs. 5 μg/L | 40–860 μg/L | Fetal mortality, neonatal mortality, | Location, calendar time, arsenic exposure | 6/9 |
| Ihrig et al. 1998c,d (C‑;C) | Bryan, TX (USA) | 119 case babies, and 267 control babies | Arsenic levels estimated from airborne emissions | > 100 vs. 0 ng/m3 | Not reported | Stillbirths | Maternal age, race/ethnicity, parity, income group, exposure as a categorical variable, and exposure–race/ethnicity interaction | 7/9 |
| Aschengrau et al. 1989 (C-C)a,d | Boston, MA (USA) | 286 cases, 1,391 controls | Arsenic levels in public drinking water | 1.4–1.9 μg/L vs. UD | UD–19 μg/L | Spontaneous abortion | Water source, maternal age, educational level, history of prior spontaneous abortion | 7/9 |
| Abbreviations: BMI, body mass index; BP, blood pressure; C-C, case–control study; CS, cross-sectional study; HDSS, health and demographic surveillance system; NA, not applicable; NOS, Newcastle–Ottawa Scale; PCO, prospective cohort study; RCO, retrospective cohort study; UD, undetected. aStudies examining low to moderate arsenic dose in the general population. bStudies examining high arsenic dose in the general population. cStudies applying biomarkers/individual-level data. dStudies applying group/ecological data. eStudies that did not control for potential confounders. | ||||||||
Arsenic exposure in the general population. Spontaneous abortion. In all 10 studies that examined the association with spontaneous abortion, 4 were excluded from our quantitative analysis because the authors did not control for potential confounders. Sen and Chaudhuri (2008) studied outcome of pregnancy in 240 married women. In women with the highest concentrations of arsenic in drinking water (501–1,200 μg/L), there was an increase in spontaneous abortion. A similar observation was noted by Ahamed et al. (2006a), Mukherjee et al. (2005), and Rahman et al. (2005). Six studies provided data for our quantitative analysis (see Supplemental Material, Table S4). All the studies reported ORs. Summary OR in populations exposed to high arsenic dose (> 50 μg/L) in groundwater showed increased association (OR = 1.98; 95% CI: 1.27, 3.10) (Figure 2A). Our finding in populations exposed to low to moderate arsenic in groundwater (Guo et al. 2003) or in public tap water (Aschengrau et al. 1989) was inconclusive (Figure 2B). Overall, the summary OR was 2.02 (95% CI: 1.40, 2.91). The direction and magnitude of the association persisted in studies applying biomarkers/individual arsenic data, prospective studies, studies adjusting for adequate potential confounders, and high-quality studies (Table 2). Figure 3A shows the dose–response relation of arsenic in drinking water and spontaneous abortion. The risk trend was not consistent across the studies. A funnel plot suggested influence of publication bias (see Supplemental Material, Figure S1A), and an adjustment with the trim and fill method did not change the strength of the overall summary OR (Table 2).
Figure 2.
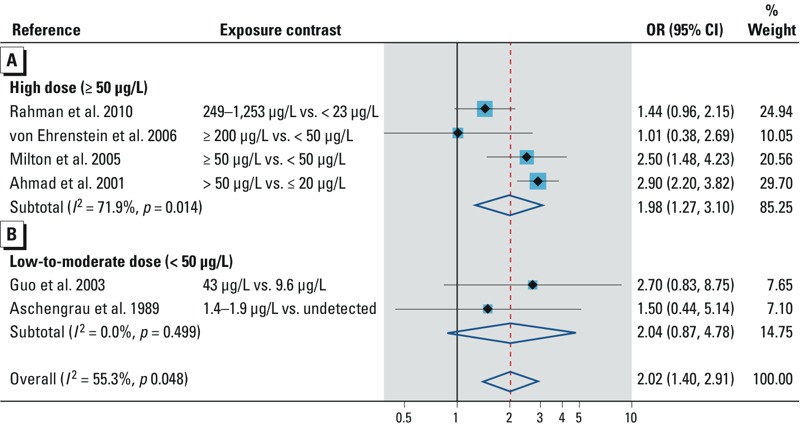
Forest plot for the relation between arsenic exposure and the risk of spontaneous abortion, assessed by (A) high arsenic dose and (B) low to moderate arsenic dose.
Table 2.
Summary OR for the relation between arsenic and the risk of adverse pregnancy/infant mortality and stratified/sensitivity analysis according to the study characteristics.
| Analysis | Spontaneous abortion | Stillbirth | Neonatal mortality | Infant mortality | ||||
|---|---|---|---|---|---|---|---|---|
| Random-effects model OR (95% CI) | Heterogeneity statistics Q (n)‑statistics, I2‑index (%), p‑value | Random-effects model OR (95% CI) | Heterogeneity statistics Q (n)‑statistics, I2‑index (%), p‑value | Random-effects model OR (95% CI) | Heterogeneity statistics Q (n)‑statistics, I2‑index (%), p‑value | Random-effects model OR (95% CI) | Heterogeneity statistics Q (n)‑statistics, I2‑index (%), p‑value | |
| Summary OR | 2.02 (1.40, 2.91) | 11.2 (6), 55.3, 0.048 | 1.84 (1.38, 2.45) | 38.40 (9), 79.2, 0.000 | 1.51 (1.28, 1.78) | 5.34 (5), 25.1, 0.254 | 1.35 (1.12, 1.62) | 8.31 (7), 30.4, 0.216 |
| Stratified analysis | ||||||||
| Assessment of arsenic exposure | ||||||||
| Individual data/biomarker | 2.20 (1.04, 3.46) | 7.96 (3), 74.9, 0.019 | 1.96 (1.17, 3.29) | 19.91 (5), 79.9, 0.001 | 1.30 (1.00, 1.67) | 4.78 (2), 16.4, 0.274 | 1.74 (0.92, 3.28) | 6.02 (3), 66.8, 0.049 |
| Group data | 1.51 (0.79, 2.87) | 1.59 (3), 0.0, 0.951 | 1.79 (1.29, 2.48) | 5.46 (4), 45.1, 0.141 | 1.59 (1.43, 1.77) | 1.75 (3), 0.0, 0.416 | 1.32 (1.08, 1.60) | 2.44 (4), 0.0, 0.486 |
| Sensitivity analysis | ||||||||
| Prospective cohort studiesa | 1.45 (0.99, 2.12) | 0.66 (2), 0.0, 0.951 | 1.13 (0.98, 1.30) | 0.67 (2), 0.0, 0.412 | 1.21 (0.98, 1.50)a | 2.12 (0.53, 8.42) | 4.84 (2), 79.3, 0.028 | |
| Studies adjusting for potential confounders | 1.72 (1.25, 2.37) | 4.43 (5), 9.7, 0.351 | 1.85 (1.22, 2.82) | 18.06 (7), 66.8, 0.005 | 1.53 (1.11, 2.10) | 4.63 (4), 35.2, 0.201 | 1.65 (1.01, 2.47) | 8.39 (5), 52.3, 0.078 |
| High-quality studies (> 7 on NOS) | 1.45 (0.99,1.12) | 1.65 (3), 0.0, 0.438 | 1.28 (0.98, 1.67) | 4.27 (4), 29.7, 0.234 | 1.49 (0.92, 2.31) | 2.57 (2), 61.1, 0.109 | 1.41 (1.04,1.92) | 7.55 (4), 60.2, 0.056 |
| Impact of missing studies on overall summary OR | ||||||||
| By trim and fill method | 2.02 (1.20, 2.91) | 55.3 (6), 55.3, 0.048 | 1.43 (1.11, 1.85) | 58.06 (13), 79.33, 0.000 | 1.47 (1.27, 1.71) | 7.15 (7), 2.15, 0.307 | 1.22 (0.98, 1.53) | 8.62 (10), 30.0, 0.017 |
| Abbreviations: NOS, Newcastle–Ottawa Scale; Q(n), n: number of studies.aOne prospective study reported on neonatal mortality. | ||||||||
Figure 3.
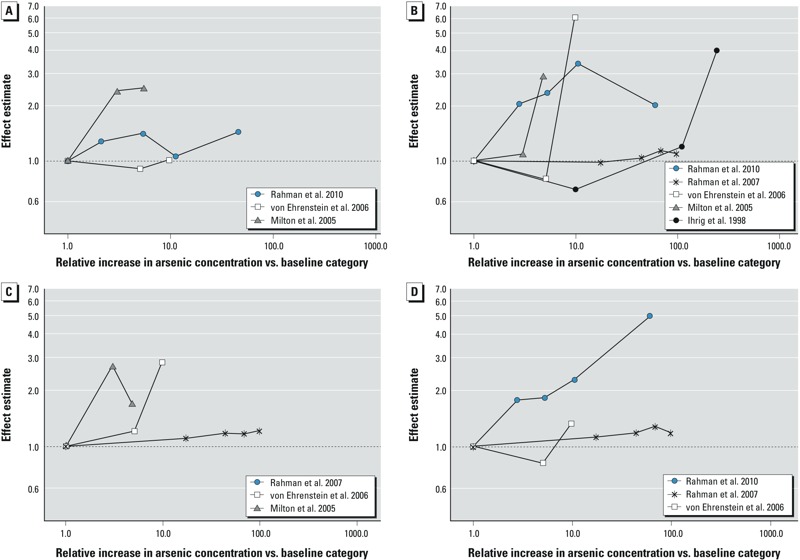
Plots of dose–response relations for arsenic and (A) spontaneous abortion, (B) stillbirth, (C) neonatal mortality, and (D) infant mortality in the general population.
Stillbirth. Of the 14 studies reporting association with stillbirth, 5 (Ahamed et al. 2006a; Chakraborti et al. 2003; Mukherjee et al. 2005; Rahman et al. 2005; Sen and Chaudhuri 2008) were excluded from our quantitative analysis because the authors did not control for potential confounders. All 5 studies observed an increase in stillbirth in women who had the highest concentrations of arsenic in their drinking water. Nine studies examining the association between environmental arsenic and stillbirth provided data for our quantitative analysis (see Supplemental Material, Table S4). Two studies (Hopenhayn-Rich et al. 2000; Rahman et al. 2007) reported RRs. Arsenic was measured in groundwater in 8 studies and in air in 1 study. Summary OR in populations exposed to high arsenic dose (> 50 μg/L) in groundwater was increased (OR = 1.77; 95% CI: 1.32, 2.36; Figure 4A). Only 1 study (Ihrig et al. 1998) was conducted in a population exposed to low-to-moderate arsenic dose (Figure 4B). The overall summary OR for environmental arsenic was 1.84 (95% CI: 1.38, 2.45). In subgroup/sensitivity analyses, the risk of stillbirth was increased in studies applying biomarkers/individual arsenic data, studies using group data on arsenic, studies adjusting for adequate potential confounders, prospective studies, and high-quality studies (Table 2). Five studies reported a dose–response relationship between environmental arsenic and stillbirth (Figure 3B). Risk trend was consistent in 2 studies in high arsenic dose area (Milton et al. 2005; Rahman et al. 2010), but this trend was not obvious in the other studies. A funnel plot suggested influence of small positive studies (see Supplemental Material, Figure S1B). The trim and fill method for adjustment of publication bias imputed 4 studies, and, as expected, the strength of the summary OR was attenuated but remained statistically significant (Table 2).
Figure 4.
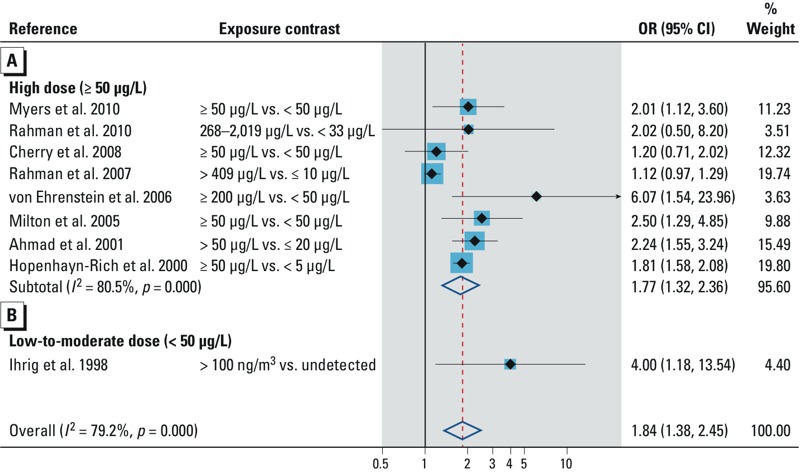
Forest plot for the relation between arsenic exposure and the risk of stillbirth, assessed by (A) high arsenic dose and (B) low to moderate arsenic dose.
Preterm delivery. In all, three studies (Ahmad et al. 2001; Myers et al. 2010; Yang et al. 2003) investigated the relation between arsenic exposure and preterm delivery (see Supplemental Material, Table S4). They all reported ORs and were conducted in populations exposed to high arsenic dose in groundwater. The finding of the summary OR was inconclusive (OR = 1.41; 95% CI: 0.83, 2.41) (Figure 5A).
Figure 5.
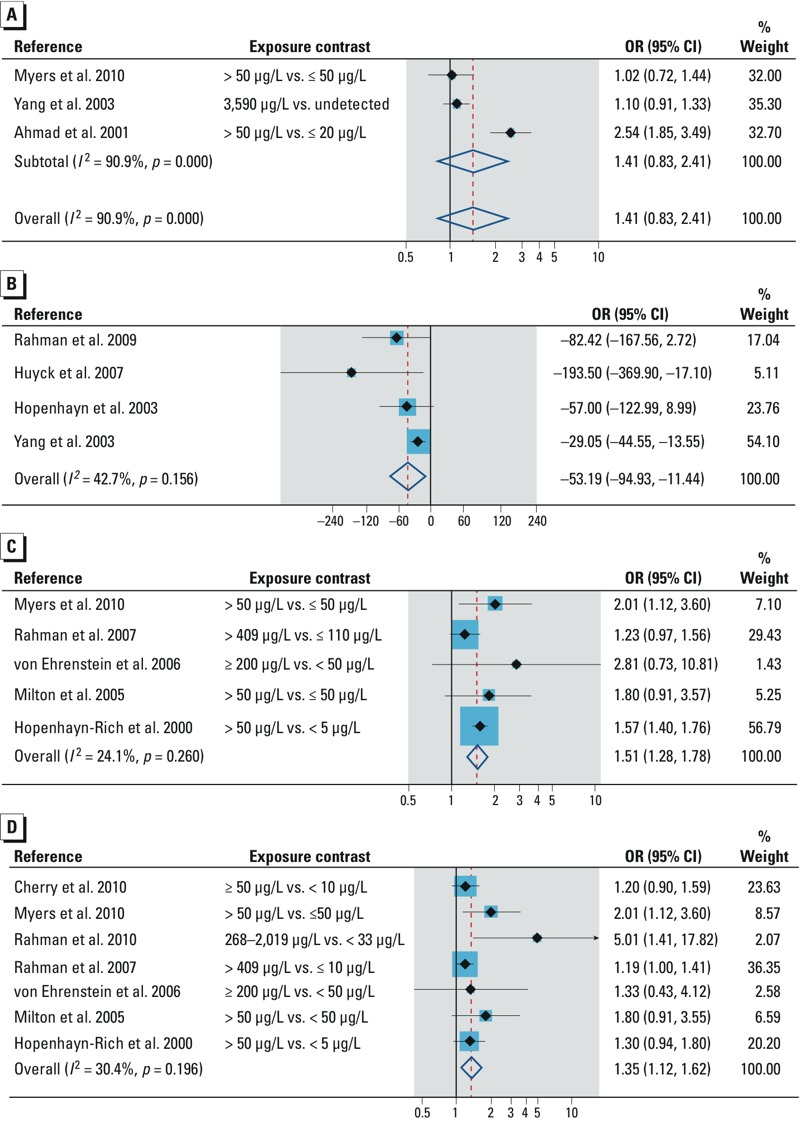
Forest plot for the relation between arsenic exposure and (A) preterm delivery, (B) birth weight, (C) neonatal mortality, and (D) infant mortality.
Birth weight. The association between arsenic and birth weight was examined in six studies. Two studies did not provide sufficient quantitative data for the meta-analysis. Fei et al. (2013) measured arsenic dose in maternal urine (UAs) and observed an inverse dose response (coefficient: β = –1.3) between UAs and birth weight. Guan et al. (2012) also observed that newborns of mothers whose UAs was > 5.30 μg/L weighed on average 0.22 kg less than those of mothers whose UAs was ≤ 5.30 μg/L. Four studies (Hopenhayn et al. 2003; Huyck et al. 2007; Rahman et al. 2009; Yang et al. 2003) provided regression coefficient and standard errors for our quantitative analysis (see Supplemental Material, Table S4). Environmental arsenic shows a significant reduction in birth weight (–53.2 g; 95% CI: –94.9, –11.4) (Figure 5B).
Neonatal mortality. Five studies examined neonatal mortality (see Supplemental Material, Table S5), of which two (Hopenhayn-Rich et al. 2000; Rahman et al. 2007) reported RRs. All the studies were conducted in populations exposed to high arsenic dose in groundwater. The overall summary OR was 1.51 (95% CI: 1.28, 1.78) (Figure 5C). The direction of association did not change in studies applying biomarkers/individual arsenic data and in studies adjusting for adequate potential confounders (Table 2). Rahman et al. (2007) reported on this relation. Dose response was examined in three studies (Figure 3C.) A consistent dose–response trend was observed by von Ehrenstein et al. (2006), but the risk trend was inconsistent in studies by Milton et al. (2005) and Rahman et al. (2007). Evidence of publication bias was observed in the funnel plot (see Supplemental Material, Figure S1C). The trim and fill method imputed two studies, and the strength of association was reduced marginally (Table 2).
Infant mortality. Arsenic and infant mortality was investigated in seven studies (see Supplemental Material, Table S5), with two (Hopenhayn-Rich et al. 2000; Rahman et al. 2007) reporting RRs. The studies were conducted in populations exposed to high arsenic dose in groundwater. Summary OR was 1.35 (95% CI: 1.12, 1.62) (Figure 5D). Compared with that of the overall summary OR, the association was slightly elevated in studies applying biomarkers/individual arsenic data, studies adjusting for adequate potential confounders, and high-quality studies, but marginally reduced in studies using group data on arsenic (Table 2). Our findings from two prospective studies were inconclusive. Among three studies examining dose response (Figure 3D), a consistent risk trend was observed by Rahman et al. (2010), but the risk trend was not consistent in studies by Rahman et al. (2007) and von Ehrenstein et al. (2006). A funnel plot showed evidence of asymmetry, suggesting influence of small positive studies (see Supplemental Material, Figure S1D). As expected, the strength of association was attenuated with the trim and fill method, and three missing studies were imputed (Table 2).
Discussion
This is the first systematic review and meta-analysis on the association between iAs exposure and adverse pregnancy outcomes/infant mortality. We found positive associations of arsenic with spontaneous abortion, stillbirth, birth weight, and neonatal and infant mortality. These findings are important to many countries around the globe where pregnant women and infants continue to be exposed to low through moderate to high arsenic dose in different media (e.g., drinking water, air, food, beverages).
Validity issues. Our study has a number of strengths. We searched several databases including reference lists of reviews and relevant studies. Two authors independently checked the eligibility of the studies according to a predefined set of criteria. We followed systematically the guideline of PRISMA (Moher et al. 2009).
Because the upper limits of arsenic exposure differed among studies, we studied the effect in populations in low-to-moderate arsenic areas (i.e., < 50 μg/L) separately from the effect in populations in high arsenic areas (i.e., ≥ 50 μg/L). In studies of spontaneous abortion and stillbirth, the findings were inconclusive.
We excluded from our quantitative analysis small ecological studies that did not adjust for potential confounders (Altman 1994; Turner et al. 2013). Also, in considering our core and additional confounders various studies should have adjusted for, we followed recommendations in the literature (e.g., Di Mario et al. 2007; Ghosh 2012; Kramer 1987, 2003; Kumar 2011; McClure et al. 2006; Moss et al. 2002; Shah et al. 2011).
While acknowledging the importance of our findings, we note a number of limitations. First, the use of summary scores to identify high-quality studies in Newcastle–Ottawa Scale is a bit problematic. A risk of bias tool that applies a domain-based evaluation may allow one to explore the influence of each domain on the overall summary effect estimate (Higgins and Green 2011; National Research Council 2004). Second, a well-designed study may be categorized as low quality because the authors failed to provide detail information in the publication. Finally, some items of the Newcastle–Ottawa Scale such as representativeness of study cohort with respect to community and duration of follow-up do not belong to the risk of bias tools (Deeks et al. 2003; National Research Council 2004; Sanderson et al. 2007). Thus, the interpretation of how well a study does on the Newcastle–Ottawa Scale in our study should be done with caution. Inclusion of ecological studies in our review may lead to underestimation of our observed associations. Also the studies incorporated in this meta-analysis were different with regard to exposure levels in the reference groups. However, in computing the overall summary OR from the different studies, we made an implicit assumption that any differences in exposure levels in the reference groups will not have much influence on our summary OR.
We observed substantial heterogeneity in the study-specific estimates for studies on spontaneous abortion and stillbirth, and moderate heterogeneity for studies on neonatal and infant mortality. In stratified analysis, heterogeneity persisted in studies applying biomarkers for the association with spontaneous abortion, stillbirth, and infant mortality. In sensitivity analyses, heterogeneity persisted in prospective studies on infant mortality, studies on stillbirth and infant mortality that have controlled for adequate potential confounders, and high-quality studies on neonatal and infant mortality. The original studies also applied different exposure assessment methods and incorporated different exposure contrasts, thus making it difficult to relate any exposure increase to change in birth weight. Differences in responses to arsenic exposure may also exist across study populations (Abhyankar et al. 2012; Concha et al. 2002; Hopenhayn-Rich et al. 1998), and these could be potential sources of the observed heterogeneity. We lacked data on these factors and we also did not have sufficient data from the original studies to elaborate further the reasons for the heterogeneity. We applied the trim and fill method to examine the impact of publication bias on our overall summary OR, and the summary OR was slightly reduced for stillbirth, neonatal mortality, and infant mortality, suggesting that publication bias is not an explanation of our observed associations. Nonetheless, the trim and fill method performs poorly in the presence of substantial heterogeneity, so the influence of publication bias on the observed associations cannot be ruled out.
Our findings, however, should be interpreted in the light of limitations inherent in the original studies. Some studies failed to adjust for appropriate potential confounders of adverse pregnancy outcomes/infant mortality and could not establish the independent role of arsenic. Although few studies adjusted for proxies of socioeconomic status, only one study considered access and utilization of prenatal care. This is an important socioeconomic factor to be considered in the studies of stillbirth and neonatal/infant mortality (Kiely et al. 1985; Ronsmans et al. 2004; Shah et al. 2011). Exposure assessment was also a major challenge in the studies. Three studies measured arsenic contents in urine (Fei et al. 2013; Rahman et al. 2007, 2010). One study measured arsenic content in blood (Guan et al. 2012), and Huyck et al. (2007) measured arsenic content in hair. Arsenic content in urine/blood is a marker of current exposure, whereas information on chronic exposure can be obtained from arsenic content in hair or finger/toe nails. The remaining studies applied ecological measures. Questionnaires were administered in most studies, but data on water consumption pattern (i.e., the frequency and quantity of water intake) were not reported. Lack of individual data may result in measurement error with underestimation of the true effect. Many of the studies were cross-sectional in design, precluding temporality. Although few studies have collected data on our outcomes of interest from medical records/established registers, most studies have relied on maternal recall. Methods applied in collecting data on spontaneous abortion were not sensitive enough to detect events occurring in early pregnancy. Thus, the fetal and infant health effect of arsenic observed in our study may have been substantially underestimated.
Comparison with previous studies. Only two qualitative reviews were available on this subject. In the first study, Smith and Steinmaus (2009) examined the effects of arsenic and chromium in drinking water on low birth weight and infant mortality. The authors identified 10 studies and failed to reach any conclusion. In the second study, Bloom et al. (2010) examined the association of spontaneous abortion with arsenic in drinking water. The authors also identified 9 studies and concluded that chronic exposure to arsenic was associated with spontaneous abortion. In the present study, we observed excess risk of 102% for spontaneous abortion, 84% for stillbirth, 51% for neonatal mortality, 35% for infant mortality, and a 53-g reduction in birth weight. The magnitude of association persisted in studies applying biomarkers, studies using aggregate data on arsenic exposure, studies adjusting for adequate potential confounders, and high-quality studies. From the global public health point of view, the observed association is relevant considering the magnitude of the estimated effect and the extent of exposure to arsenic globally.
The precise biologic window of susceptibility of arsenic for adverse pregnancy outcomes is unknown (Vahter 2007, 2009). But arsenic exposure at different periods before or during pregnancy could cause a wide range of adverse pregnancy outcomes (Selevan et al. 2000). In laboratory animals, prenatal arsenic exposure causes spontaneous abortion by defective implantation or zygote development and aneuploidy, or through aberrant placental vasculogenesis and placental insufficiency (He et al. 2007; Navarro et al. 2004). Epidemiologic studies have also shown that arsenic causes oxidative stress, lipid peroxidation, interference of hormonal activities, and perturbation of DNA methylation, which may be associated with a wide range of adverse pregnancy outcomes through defective placentation and preeclampsia (Concha et al. 1998; Hood et al. 1988; Hu et al. 1998; Hughes 2002; Vahter 2007, 2009).
Our findings suggest that the effect of arsenic is strongest for spontaneous abortion. Although methylation is expected to have improved dramatically in the second trimester (Concha et al. 1998; Vahter 2007), at high arsenic dose (≥ 50 μg/L) observed in the populations included in our study, methylation is inhibited and the fetus blood plasma may essentially contain unmethylated arsenic and monomethylarsonic acid, which could threatened fetal survival and growth (Hall et al. 2013; Vahter 2007). Exposure to arsenic in utero and in early life may also pose a threat to infant survival (Hughes 2002; Vahter 2007). This observation has been noted in series of cohort studies conducted in the developing countries (Milton et al. 2005; Myers et al. 2010; Rahman et al. 2009, 2010).
Studies with the greatest weight in the meta-analyses did not provide data for the evaluation of dose–response trend. However, in the few studies that provided data, we observed inconsistent dose–response trend at high arsenic dose. The evidence was scarce for low to moderate arsenic dose and for studies evaluating preterm delivery.
Conclusions
Our systematic review and meta-analysis found positive associations of arsenic exposure with spontaneous abortion, stillbirth, birth weight, and neonatal and infant mortality. However, the interpretation of causal association of high arsenic dose in drinking water is limited by methodological problems in the original studies and limited studies on dose response.
Supplemental Material
Footnotes
The authors declare they have no actual or potential competing financial interests.
References
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A.2012Arsenic exposure and hypertension: a systematic review. Environ Health Perspect 120494–500.; 10.1289/ehp.1103988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed S, Sengupta MK, Mukherjee A, Hossain MA, Das B, Nayak B, et al. Arsenic groundwater contamination and its health effects in the state of Uttar Pradesh (UP) in upper and middle Ganga plain, India: a severe danger. Sci Total Environ. 2006a;370:310–322. doi: 10.1016/j.scitotenv.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Ahamed S, Sengupta MK, Mukherjee SC, Pati S, Mukherjee A, Rahman MM, et al. An eight-year study report on arsenic contamination in groundwater and health effects in Eruani village, Bangladesh and an approach for its mitigation. J Health Popul Nutr. 2006b;24:129–241. [PubMed] [Google Scholar]
- Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG. The scandal of poor medical research [Editorial]. BMJ. 1994;308:283–284. doi: 10.1136/bmj.308.6924.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschengrau A, Zierler S, Cohen A. Quality of community drinking water and the occurrence of spontaneous abortion. Arch Environ Health. 1989;44:283–290. doi: 10.1080/00039896.1989.9935895. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Fitzgerald EF, Kim K, Neamtiu I, Gurzau ES. Spontaneous pregnancy loss in humans and exposure to arsenic in drinking water. Int J Hyg Environ Health. 2010;213:401–413. doi: 10.1016/j.ijheh.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. New York: JohnWiley and Sons; 2009. Introduction to Meta-Analysis. [Google Scholar]
- Chakraborti D, Mukherjee SC, Pati S, Sengupta MK, Rahman MM, Chowdhury UK, et al. 2003Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger? Environ Health Perspect 1111194–1201.; 10.1289/ehp.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry N, Shaikh K, McDonald C, Chowdhury Z. Stillbirth in rural Bangladesh: arsenic exposure and other etiological factors: a report from Gonoshasthaya Kendra. Bull World Health Organ. 2008;86:172–177. doi: 10.2471/BLT.07.043083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry N, Shaik K, McDonald C, Chowdhury Z. Manganese, arsenic, and infant mortality in Bangladesh: an ecological analysis. Arch Environ Occup Health. 2010;65:148–153. doi: 10.1080/19338240903390362. [DOI] [PubMed] [Google Scholar]
- Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- Concha G, Vogler G, Nermell B, Vahter M. Intra-individual variation in the metabolism of inorganic arsenic. Int Arch Occup Environ Health. 2002;75:576–580. doi: 10.1007/s00420-002-0361-1. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- Di Mario S, Say L, Lincetto O. Risk factors for stillbirth in developing countries: a systematic review of the literature. Sex Transm Dis. 2007;34(7 suppl):S11–S21. doi: 10.1097/01.olq.0000258130.07476.e3. [DOI] [PubMed] [Google Scholar]
- Essumang DK. Analysis and human health risk assessment of arsenic, cadmium, and mercury in Manta birostris (manta ray) caught along the Ghanaian coastline. Hum Ecol Risk Assess. 2009;15:985–998. [Google Scholar]
- Essumang DK, Dodoo DK, Obiri S, Yaney JY. Arsenic, cadmium, and mercury in cocoyam (Xanthosoma sagititolium) and watercocoyam (Colocasia esculenta) in Tarkwa a mining community. Bull Environ Contam Toxicol. 2007;79:377–379. doi: 10.1007/s00128-007-9244-1. [DOI] [PubMed] [Google Scholar]
- Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, et al. 2013Association between in utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environ Health 1258; 10.1186/1476-069X-12-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Sancha AM. Arsenic exposure and its impact on health in Chile. J Health Popul Nutr. 2006;24:164–175. [PubMed] [Google Scholar]
- Ghosh R. Child mortality in India: a complex situation. World J Pediatr. 2012;8:11–18. doi: 10.1007/s12519-012-0331-y. [DOI] [PubMed] [Google Scholar]
- Guan H, Piao F, Zhang X, Li X, Li Q, Xu L, et al. Prenatal exposure to arsenic and its effects on fetal development in the general population of Dalian. Biol Trace Elem Res. 2012;149:10–15. doi: 10.1007/s12011-012-9396-7. [DOI] [PubMed] [Google Scholar]
- Guo X, Fujino Y, Chai J, Wu K, Xia Y, Li Y, et al. The prevalence of subjective symptoms after exposure to arsenic in drinking water in Inner Mongolia, China. J Epidemiol. 2003;13:211–215. doi: 10.2188/jea.13.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, Slavkovich V, et al. 2013Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect 1211068–1074.; 10.1289/ehp.1205727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, et al. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40:1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- He W, Greenwell RJ, Brooks DM, Calderón-Garcidueñas L, Beall HD., Coffin JD. Arsenic exposure in pregnant mice disrupts placental vasculogenesis and causes spontaneous abortion. Toxicol Sci. 2007;99:244–253. doi: 10.1093/toxsci/kfm162. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. 2011. Available: http://www.cochrane.org/handbook [accessed 22 June 2012]
- Hood RD, Vedel GC, Zaworotko MJ, Tatum FM, Meeks RG. Uptake, distribution, and metabolism of trivalent arsenic in the pregnant mouse. J Toxicol Environ Health. 1988;25:423–434. doi: 10.1080/15287398809531221. [DOI] [PubMed] [Google Scholar]
- Hopenhayn C, Bush HM, Bingcang A, Hertz-Picciotto I. Association between arsenic exposure from drinking water and anemia during pregnancy. J Occup Environ Med. 2006;48:635–643. doi: 10.1097/01.jom.0000205457.44750.9f. [DOI] [PubMed] [Google Scholar]
- Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, et al. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14:593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Córdoba, Argentina. Int J Epidemiol. 1998;27:561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, Ferreccio C, Peralta C, Gibb H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ Health Perspect. 2000;108:667–673. doi: 10.1289/ehp.00108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Su L, Snow ET. Arsenic toxicity is enzyme specific and its affects on ligation are not caused by the direct inhibition of DNA repair enzymes. Mutat Res. 1998;408:203–218. doi: 10.1016/s0921-8777(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Hughes MF.2006Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect 1141790–1796.; 10.1289/ehp.9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49:1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- Ihrig MM, Shalat SL, Baynes C. A hospital-based case-control study of stillbirths and environmental exposure to arsenic using an atmospheric dispersion model linked to a geographical information system. Epidemiology. 1998;9:290–294. [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risk Hum. 2004;84:1–477. [PMC free article] [PubMed] [Google Scholar]
- Kiely JL, Paneth N, Susser M. Fetal death during labor: an epidemiologic indicator of level of obstetric care. Am J Obstet Gynecol. 1985;153:721–727. doi: 10.1016/0002-9378(85)90331-x. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133(5 suppl 2):1592S–1596S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- Kumar S. Occupational, environmental and lifestyle factors associated with spontaneous abortion. Reprod Sci. 2011;18:915–930. doi: 10.1177/1933719111413298. [DOI] [PubMed] [Google Scholar]
- Kwok RK. A review and rationale for studying the cardiovascular effects of drinking water arsenic in women of reproductive age. Toxicol Appl Pharmacol. 2007;222:344–350. doi: 10.1016/j.taap.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Landgren O. Environmental pollution and delivery outcome in southern Sweden: a study with central registries. Acta Paediatr. 1996;85:1361–1364. doi: 10.1111/j.1651-2227.1996.tb13926.x. [DOI] [PubMed] [Google Scholar]
- McClure EM, Nalubamba-Phiri M, Goldenberg RL. Stillbirth in developing countries. Int J Gynaecol Obstet. 2006;94:82–90. doi: 10.1016/j.ijgo.2006.03.023. [DOI] [PubMed] [Google Scholar]
- McDonald C, Hoque R, Huda N, Cherry N. Risk of arsenic-related skin lesions in Bangladeshi villages at relatively low exposure: a report from Gonoshasthaya Kendra. Bull World Health Organ. 2007;85:668–673. doi: 10.2471/BLT.06.036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AH, Smith W, Rahman B, Hasan Z, Kulsum U, Dear K, et al. Chronic arsenic exposure and adverse pregnancy outcomes in Bangladesh. Epidemiology. 2005;16:82–86. doi: 10.1097/01.ede.0000147105.94041.e6. [DOI] [PubMed] [Google Scholar]
- Mink PJ, Alexander DD, Barraj LM, Kelsh MA, Tsuji JS. Low-level arsenic exposure in drinking water and bladder cancer: a review and meta-analysis. Regul Toxicol Pharmacol. 2008;52:299–310. doi: 10.1016/j.yrtph.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Ann Intern Med. 2013;159:649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss W, Darmstadt GL, Marsh DR, Black RE, Santosham M. Research priorities for the reduction of perinatal and neonatal morbidity and mortality in developing country communities. J Perinatol. 2002;22:484–495. doi: 10.1038/sj.jp.7210743. [DOI] [PubMed] [Google Scholar]
- Mukherjee SC, Saha KC, Pati S, Dutta RN, Rahman MM, Sengupta MK, et al. Murshidabad—one of the nine groundwater arsenic-affected districts of West Bengal, India. Part II: dermatological, neurological, and obstetric findings. Clin Toxicol (Phila) 2005;43:835–848. doi: 10.1080/15563650500357495. [DOI] [PubMed] [Google Scholar]
- Murcott S. London: IWA Publishing; 2012. Arsenic Contamination in the World: An International Sourcebook 2012. [Google Scholar]
- Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, et al. Maternal drinking water arsenic exposure and perinatal outcomes in Inner Mongolia, China. J Epidemiol Commun Health. 2010;64:325–329. doi: 10.1136/jech.2008.084392. [DOI] [PubMed] [Google Scholar]
- National Research Council. Washington, DC: National Academies Press; 2004. Review of EPA’s Integrated Risk Information System (IRIS) Process. [PubMed] [Google Scholar]
- Navarro PA, Liu L, Keefe DL. In vivo effects of arsenite on meiosis, preimplantation development, and apoptosis in the mouse. Biol Reprod. 2004;70:980–985. doi: 10.1095/biolreprod.103.020586. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Nachman KE. Public health responses to arsenic in rice and other foods. JAMA Intern Med. 2013;173:1395–1396. doi: 10.1001/jamainternmed.2013.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162:1037–1049. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E.2006Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect 114641–648.; 10.1289/ehp.8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiri S, Dodoo DK, Essumang DK, Armah FA. Cancer and non-cancer risk assessment from exposure to arsenic, copper, and cadmium in borehole, tap, and surface water in the Obuasi Municipality, Ghana. Human Eco Risk Assess. 2010;16:651–665. [Google Scholar]
- Quansah R, Jaakkola JJ. Occupational exposures and adverse pregnancy outcomes among nurses: a systematic review and meta-analysis. J Womens Health (Larchmt) 2010;19:1851–1862. doi: 10.1089/jwh.2009.1876. [DOI] [PubMed] [Google Scholar]
- Rahman A, Persson LÅ, Nermell B, El Arifeen S, Ekström EC, Smith AH, et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21:797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Ekström EC, Rahman M, Golam Mustafa AH, Wahed MA, et al. Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh. Am J Epidemiol. 2007;165:1389–1396. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169:304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Sengupta MK, Ahamed S, Chowdhury UK, Lodh D, Hossain A, et al. Arsenic contamination of groundwater and its health impact on residents in a village in West Bengal, India. Bull World Health Organ. 2005;83:49–57. [PMC free article] [PubMed] [Google Scholar]
- Ronsmans C, De Brouwere V, Dubourg D, Dieltiens G. Measuring the need for life-saving obstetrics surgery in developing countries. BJOG. 2004;111:1027–1030. doi: 10.1111/j.1471-0528.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen J, Chaudhuri AB. Arsenic exposure through drinking water and its effect on pregnancy outcome in Bengali women. Arh Hig Rada Toksikol. 2008;59:271–275. doi: 10.2478/10004-1254-59-2008-1871. [DOI] [PubMed] [Google Scholar]
- Shah PS, Zao J, Al-Wassia H, Shah V, Knowledge Synthesis Group on Determinants of Preterm/LBW Births. Pregnancy and neonatal outcomes of aboriginal women: a systematic review and meta-analysis. Womens Health Issues. 2011;21:28–39. doi: 10.1016/j.whi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107–122. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Moore L, Hopenhayn-Rich C, Biggs ML, Smith AH. Arsenic in drinking water and bladder cancer. Cancer Invest. 2000;18:174–182. doi: 10.3109/07357900009038249. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Bates MN, Smith AH. Case–control study of bladder cancer and drinking water arsenic in the western United States. Am J Epidemiol. 2003;158:1193–1201. doi: 10.1093/aje/kwg281. [DOI] [PubMed] [Google Scholar]
- Turner RM, Bird SM, Higgins JP.2013The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 8e59202; 10.1371/journal.pone.0059202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter ME. Interactions between arsenic-induced toxicity and nutrition in early life. J Nutr. 2007;137:2798–2804. doi: 10.1093/jn/137.12.2798. [DOI] [PubMed] [Google Scholar]
- Vahter ME. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Guha Mazumder DN, Hira-Smith M, Ghosh N, Yuan Y, Windham G, et al. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. Am J Epidemiol. 2006;163:662–669. doi: 10.1093/aje/kwj089. [DOI] [PubMed] [Google Scholar]
- Walvekar RR, Kane SV, Nadkarni MS, Bagwan IN, Chaukar DA, D’Cruz AK. Chronic arsenic poisoning: a global health issue—a report of multiple primary cancers. J Cutan Pathol. 2007;34:203–206. doi: 10.1111/j.1600-0560.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2009. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm [accessed 6 February 2011]
- WHO (World Health Organization). Geneva: WHO; 2011. Guidelines for Drinking-Water Quality. 4th ed. [Google Scholar]
- Yang CY, Chang CC, Tsai SS, Chuang HY, Ho CK, Wu TN. Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ Res. 2003;91:29–34. doi: 10.1016/s0013-9351(02)00015-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


