Abstract
Background: The exposome encompasses all life-course environmental exposures from the prenatal period onward that influence health. MicroRNAs (miRNAs) are interesting entities within this concept as markers and causation of disease. MicroRNAs are short oligonucleotide sequences that can interact with several mRNA targets.
Objectives: We reviewed the current state of the field on the potential of using miRNAs as biomarkers for environmental exposure. We investigated miRNA signatures in response to all types of environmental exposure to which a human can be exposed, including cigarette smoke, air pollution, nanoparticles, and diverse chemicals; and we examined the health conditions for which the identified miRNAs have been reported (i.e., cardiovascular disease, cancer, and diabetes).
Methods: We searched the PubMed and ScienceDirect databases to identify relevant studies.
Results: For all exposures incorporated in this review, 27 miRNAs were differentially expressed in at least two independent studies. miRNAs that had expression alterations associated with smoking observed in multiple studies are miR-21, miR-34b, miR-125b, miR-146a, miR-223, and miR-340; and those miRNAs that were observed in multiple air pollution studies are miR-9, miR-10b, miR-21, miR-128, miR-143, miR-155, miR-222, miR-223, and miR-338. We found little overlap among in vitro, in vivo, and human studies between miRNAs and exposure. Here, we report on disease associations for those miRNAs identified in multiple studies on exposure.
Conclusions: miRNA changes may be sensitive indicators of the effects of acute and chronic environmental exposure. Therefore, miRNAs are valuable novel biomarkers for exposure. Further studies should elucidate the role of the mediation effect of miRNA between exposures and effect through all stages of life to provide a more accurate assessment of the consequences of miRNA changes.
Citation: Vrijens K, Bollati V, Nawrot TS. 2015. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ Health Perspect 123:399–411; http://dx.doi.org/10.1289/ehp.1408459
Introduction
Most common diseases result from the combined effect of genes and environmental factors and the interactions between them. Epigenetic effects and non-coding gene products have gained research focus over the last two decades because protein-coding genes cannot account for all observed genomic effects. Here we focus on microRNAs (miRNAs) as key regulators of health and disease. miRNAs are endogenous, single-stranded, short non-coding RNA sequences (~ 22 nucleotides) that regulate gene expression at the posttranscriptional level. Since the first discovery of miRNAs in Caenorhabditis elegans (Lee et al. 1993), hundreds of miRNAs in eukaryotes have been identified to influence physiological processes such as development, growth, differentiation, immune reaction, and adaptation to stress (van Rooij et al. 2007; Xiao et al. 2007). Diverse disease states, such as cancer and heart failure, are associated with distinct miRNA signatures, suggesting that specific miRNA programs are activated in pathophysiological processes (Calin et al. 2005).
Recent advances in molecular biology opened the opportunity for new approaches in population-based studies, in which exposures to a broad spectrum of environmental pollutants are evaluated in concert with biological systems, a concept proposed as the “exposome” (Wild 2005). From this viewpoint, miRNAs could potentially be novel biomarkers of exposure. For the purpose of this review, we focused on the response of miRNAs to environmental exposures.
miRNA characteristics. miRNA-mediated gene silencing is accomplished by base pairing of the 5´ region of miRNAs with the target mRNA sequence, leading to translational repression and/or mRNA degradation (Ambros 2004). miRNAs have been paradoxically shown to up-regulate gene expression by enhancing translation under specific conditions (Vasudevan et al. 2007). The effect of miRNA expression on gene expression is not linear, as multiple miRNAs may target the same mRNA, and the majority of mRNAs contain multiple binding sites for miRNAs, generating a highly complex regulatory network system (Saetrom et al. 2007). For details on miRNA synthesis, biogenesis, miRNA mechanism of action, see Figure 1 and reviews by Djuranovic et al. (2011) and Murchison and Hannon (2004).
Figure 1.
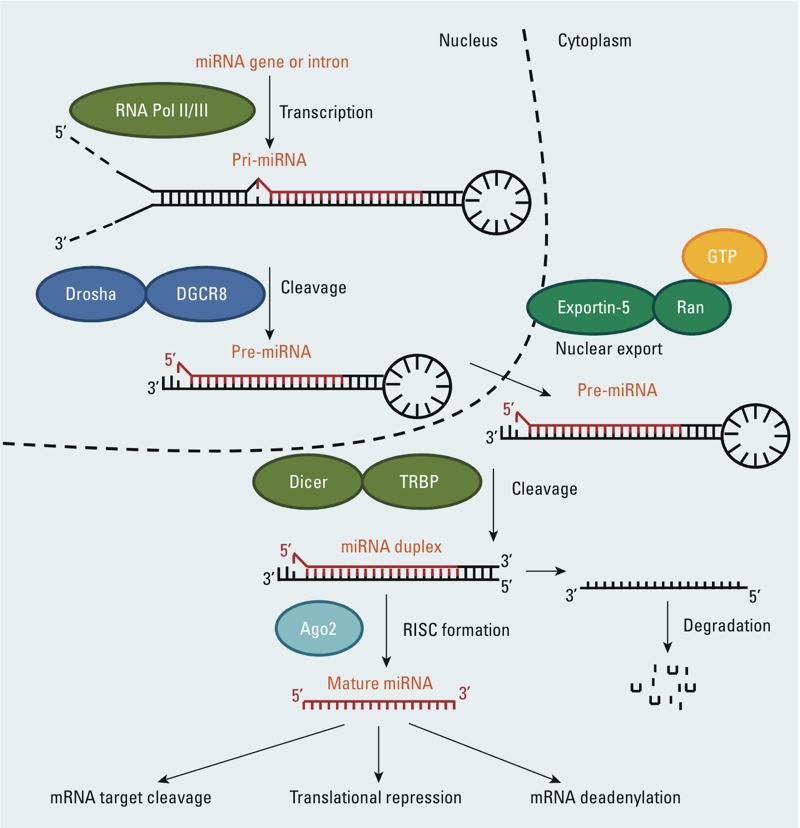
Overview of miRNA biogenesis. The canonical maturation of a miRNA includes the production of the primary miRNA transcript (pri-miRNA) by RNA polymerase II or III (Pol II/III) and cleavage of the pri-miRNA by the microprocessor complex Drosha–DGCR8 (Pasha) in the nucleus. The resulting precursor hairpin, the pre-miRNA, is exported from the nucleus by Exportin-5–Ran-GTP. In the cytoplasm, the RNase Dicer in complex with the double-stranded RNA-binding protein TRBP cleaves the pre-miRNA hairpin to its mature length. The functional strand of the mature miRNA is loaded together with Argonaute (Ago2) proteins into the RNA-induced silencing complex (RISC), where it guides RISC to silence target mRNAs through mRNA cleavage, translational repression, or deadenylation, whereas the passenger strand (black) is degraded.
miRNA nomenclature. miRNAs are named using the “miR” prefix and a unique identifying number (e.g., miR-1, miR-2). The identifying numbers are assigned sequentially, with identical miRNAs having the same number, regardless of organism. Paralogous sequences whose mature miRNAs differ at only one or two positions are given lettered suffixes: for example, miR-10a and miR-10b. Distinct hairpin loci that give rise to identical mature miRNAs have numbered suffixes (e.g., mir-281-1, mir-281-2). The mature sequences are designated “miR,” whereas the precursor hairpins are labeled “mir.” The -3p and -5p suffixes that sometimes appear within an miR name refer to the arm from which the mature sequence comes. For nomenclature guidelines, see Ambros et al. (2003).
miRNA analysis techniques suitable for large epidemiological studies. In recent years, miRNA expression changes following exposure to environmental toxicants, even before disease onset, have gained researchers’ interest. The measure of miRNAs in large epidemiological studies needs to be high throughput and sensitive enough to detect small changes in healthy subjects. At the same time, techniques need to be affordable in order to be conducted in large population studies. Moreover, given the complexity of phenomena induced by exposure but not fully explained by an effect on a single transcript, current research is going toward genome-wide techniques. Another challenge is tissue specificity of miRNAs: The availability of only noninvasive samples in epidemiological studies conducted on healthy populations limits our capability to investigate target tissues and opens important questions on the meaning of those markers in surrogate tissues. In epidemiological research, free and exosomal miRNAs in body fluids are interesting study objects because of their potential to serve as a proxy for tissue-specific miRNAs. A limitation of this approach is that these miRNAs differ between different body fluids, and their function is not clear. Although miRNAs hold promise as exposure biomarkers, recent studies have been primarily disease focused [reviewed by Etheridge et al. (2011)].
Genome-wide miRNA analysis can be achieved by amplification-based [real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR)], hybridization-based (microarrays), and sequencing-based [next-generation sequencing (NGS)] technologies. Method selection depends on the type of sample to be analyzed and the RNA preparation protocol used. qRT-PCR is considered the gold standard because of its sensitivity, specificity, accuracy, and simple protocols. qRT-PCR can evaluate candidate miRNA expression or array plates that include a large number of miRNAs in one reaction, to OpenArray® (Applied Biosystems, Life Technologies), which allows the simultaneous amplification of a very large panel of miRNAs using nanoscale volumes. In a recent review, Prokopec et al. (2013) compared qRT-PCR to different array-based platforms used to study mRNAs/miRNAs.
Several miRNA microarray chip platforms that are commercially available [e.g., Affymetrix GeneChip® 3.0 miRNA array (Affymetrix Inc.), Agilent Human miRNA Microarray system (Agilent Technologies), Exiqon miRCURY LNA™ microarray (Exiqon Inc.)] differ in probe design and detection stringency. The limitation of this microarray chip method is the availability and stringency of probes on the chip platform that pair with miRNAs of interest. Microarrays have the advantage of being easily correlated to mRNA expression data, thus providing functional information. Furthermore, unlike other current miRNA analysis techniques, microarrays allow fast analysis of miRNAs without an arbitrary preselection step. However, the large amount of data produced can generate false-positive results, and the time-consuming step of validation by qRT-PCR is almost necessary.
NGS strategies based on deep sequencing overcome some of the technical drawbacks of probe-based methodologies, especially the ability to detect only previously known sequences (Schulte et al. 2010). As miRNAs are sequenced directly, information about sequence variations or posttranscriptional RNA editing becomes available for further analysis. The newly developed Nanostring nCounter 27 (Nanostring Technologies Inc.) uses two sequence-specific capture probes to allow for discrimination between similar variants of a single miRNA. NGS technologies [e.g., Illumina/Solexa (Illumina Inc.), GA Roche/454 GS FLX Titanium (Roche Diagnostics Corp.), and ABI/SOLID (Applied Biosystems)] allow complete “miRnomes” to be sequenced and allow for the discovery of novel miRNAs and isoforms. Another benefit of NGS technology is that it can identify precursor and primary miRNAs as well as their mature forms. NGS will likely become the gold standard for miRNA analysis because of its ability to sequence short fragments in a high-throughput mode. The choice between these methods is a key factor in establishing the possibility of success of any epidemiological study. Each method has pros and cons and should be evaluated based on the specific research.
Methods
Search strategy and selection criteria. To identify the articles relevant to this topic, we undertook a comprehensive search of the PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and ScienceDirect (http://www.sciencedirect.com/) databases initially using “microRNA” and “environmental exposure” as key terms. We did additional searches in which we replaced “microRNA” with “mir,” “miRNA,” or “epigenetic changes” and we substituted “environmental exposure” with “smoking,” “passive smoking,” “cigarette smoke,” “air pollution,” “nanoparticle exposure,” “bisphenol A,” “endocrine disruptors,” or “chemical exposure” in every possible combination. We also considered review articles as well as references found in our literature search. We excluded articles not written in English. The PubMed search covered 1 January 1980 to 1 June 2014. Articles dealing only with the description of single nucleotide polymorphisms (SNPs) in miRNA genes were disregarded, as were those articles dealing only with the description of miRNAs in nonmammalian species. A flowchart detailing the search strategy is presented in Figure 2. For miRNAs differentially expressed in response to more than one personal or environmental exposure, we researched disease phenotypes correlated with them by searching each of these miRNAs on the Human microRNA Disease Database (HMDD; http://202.38.126.151/hmdd/mirna/md/) and the miR2Disease Base (http://www.mir2disease.org/). Results of these searches are presented in Table 1, including the direction of regulation (up or down) of the miRNA and the ensuing phenotype.
Figure 2.
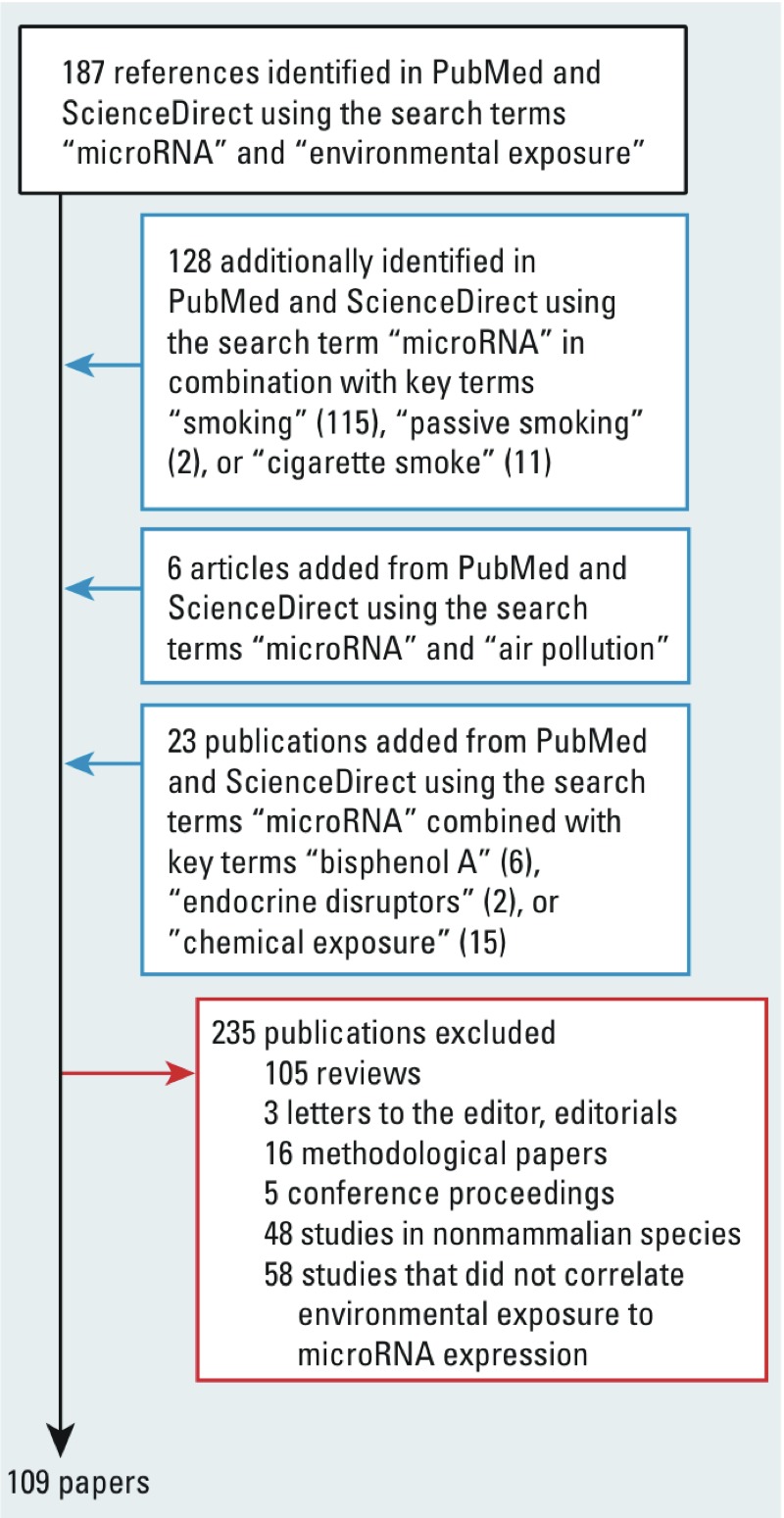
Flowchart of included studies.
Table 1.
miRNAs that are responsive to personal or environmental exposure and their roles in human disease.
| miRNA | Regulated | Exposure | Diseases | Sources |
|---|---|---|---|---|
| Let-7e | Down | TCDD | HCC, lung, pituitary, and breast cancer, GEP tumors | Feitelson and Lee 2007; Qian et al. 2009; Rahman et al. 2009; Sakurai et al. 2012; Takamizawa et al. 2004 |
| Up | RDX | Heart failure, asthma | Polikepahad et al. 2010; Thum et al. 2007 | |
| Let-7g | Down | BPA, PM | Lung carcinoma, GEP tumors, breast cancer | Rahman et al. 2009; Sakurai et al. 2012 |
| miR-9 | Down | PM | Brain cancer, Huntingon’s disease | Ferretti et al. 2009; Lau and de Strooper 2010 |
| Up | Aluminum | Hodgkin lymphoma, breast cancer | Leucci et al. 2012; Ma et al. 2010 | |
| miR-10b | Down | Formaldehyde, PM | Gastric cancer | Kim K et al. 2011 |
| miR-21 | Down | Smoking | Diabetes type 2 | Zampetaki et al. 2010 |
| Up | DEP, metal-rich PM | Breast cancer, glioblastoma, neo-intimal lesions, cardiac hypertrophy, atherosclerosis | Ji et al 2007; Raitoharju et al. 2011; van Rooij et al. 2007; Volinia et al. 2006 | |
| miR-26b | Down | DEP, BPA, PFOA | Schizophrenia, CRC, breast cancer | Earle et al. 2010; Liu et al. 2011; Perkins et al. 2007 |
| miR-31 | Down | DEP, TCDD | Medulloblastoma, T-cell leukemia | Ferretti et al. 2009; Yamagishi et al. 2012 |
| miR-34b | Down | Smoking (2×) | CRC, pancreatic, mammary, ovarian, and renal cell carcinoma | Vogt et al. 2011 |
| miR-92b | Down | Smoking, DDT | Medulloblastoma | Genovesi et al. 2011 |
| miR-122 | Down | Smoking | HCC | Bai et al. 2009 |
| Up | TCDD | Hepatitis C, renal cell carcinoma, male infertility, sepsis, hyperlipidemia | Gao et al. 2012; Henke et al. 2008; Wang C et al. 2011; Wang H et al. 2012; White et al. 2011 | |
| miR-125b | Down | Smoking (2×) | Breast cancer, head and neck cancer | Nakanishi et al. 2014; Zhang et al. 2011 |
| Up | Aluminum sulfate (2×) | Endometriosis, cardiac hypertrophy, Alzheimer’s disease | Busk and Cirera 2010; Lukiw and Alexandrov 2012; Ohlsson Teague et al. 2009 | |
| miR-135b | Down | DEP | Medulloblastoma | Lv et al. 2012 |
| Up | Smoking | CRC | Nagel et al. 2008 | |
| miR-142 | Down | Formaldehyde | Heart failure | Voellenkle et al. 2010 |
| Up | Smoking | B-cell ALL | Ju et al. 2009 | |
| miR-143 | Up | PM, ozone | Colon cancer | Zhang et al. 2013 |
| miR-146a | Down | Smoking | Postpartum psychosis, type 2 diabetes | Weigelt et al. 2013; Zampetaki et al. 2010 |
| Up | BPA, aluminum sulfate (2×) | Alzheimer’s disease, Creutzfeldt-Jakob disease, atherosclerosis, leukemia, protection against myocardial injury | Lukiw and Alexandrov 2012; Lukiw et al. 2011; Raitoharju et al. 2011; Wang et al. 2013; Wang Y et al. 2010 | |
| miR-149 | Up | BPA, DDT | Melanoma | Jin et al. 2011 |
| miR-155 | Down | PM | Hypertension | Xu et al. 2008 |
| Up | PM | Breast cancer, Hodgkin lymphoma, B-ALL | Chang et al. 2011; Kong et al. 2014; Palma et al. 2014 | |
| miR-181a | Down | Formaldehyde | Leukemia, glioma, NSCLC, breast cancer, metabolic syndrome, and CAD | Gao et al. 2010; Hulsmans et al. 2012; Marcucci et al. 2008; Ota et al. 2011; Shi et al. 2008 |
| Up | TCDD | Severe preeclampsia, male infertility | Hu et al. 2009; Wang C et al. 2011 | |
| miR-203 | Down | Smoking, formaldehyde | Myeloma | Wong et al. 2011 |
| miR-205 | Up | Smoking (2×) | Heart failure, lung cancer | Thum et al 2007; Yanaihara et al. 2006 |
| miR-206 | Up | Smoking, RDX | Myocardial infarct, slows ALS progression, myotonic dystrophy | Gambardella et al. 2010; Shan et al. 2009; Williams et al. 2009 |
| miR-222 | Up | Metal-rich PM, BPA | Severe preeclampsia, thyroid carcinoma, prostate cancer, breast cancer | Hu et al. 2009; Miller et al. 2008; Pallante et al. 2006 |
| miR-223 | Down | Smoking | AML | Eyholzer et al. 2010 |
| Up | Smoking | Heart failure, atherosclerosis | Greco et al. 2012; Kin et al. 2012 | |
| miR-338-5p | Down | Formaldehyde | Melanoma | Caramuta et al. 2010 |
| Up | DEP | Oral carcinoma | Scapoli et al. 2010 | |
| miR-340 | Down | Smoking | NA | NA |
| Up | Smoking | Heart failure, breast cancer | Thum et al. 2007; Wu et al. 2011 | |
| miR-638 | Up | BPA, DDT, arsenic | Lupus nephritis | Dai et al. 2009 |
| miR-663 | Up | BPA, DDT, arsenic | CTCL, nasopharyngeal carcinoma, burns | Liang et al. 2012; Ralfkiaer et al. 2011; Yi et al. 2012 |
| Abbreviations: ACC, acute lymphocytic leukemia; ALS, amyotrophic lateral sclerosis; AML, acute myeloid leukemia; B-ALL, B-cell acute lymphocytic leukemia; BPA, bisphenol A; CAD, coronary artery disease; CRC, colorectal carcinoma; CTCL, cutaneous T-cell lymphoma; DDT, dichlorodiphenyltrichloroethane; DEP, diesel exhaust particles; GEP, gastroenteropancreatic; HCC, hepatocellular carcinoma; NA, not applicable; NSCLC, non-small cell lung carcinoma; PFOA, perfluorooctanoic acid; PM, particulate matter; RDX, hexahydro-1,3,5-trinitro-s-triazine; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin. | ||||
Results
Smoking-induced changes in miRNA expression. The most studied environmental factor in relation to epigenetics is smoking; it was among the first factors shown to affect the miRNA machinery in humans (Spira et al. 2004). Results of in vitro studies concerning smoking and miRNAs are summarized in Table 2.
Table 2.
In vitro studies on the effects of smoking on differential miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Source |
|---|---|---|---|---|
| miR-15a | Tumor suppressor | Down | Primary bronchial epithelial cells | Schembri et al. 2009 |
| miR-125b | Targets p53, stress response | |||
| miR‑199b | Oncogene activation | |||
| miR-218 | Tumor suppressor | |||
| miR-31 | Apoptosis, tumor suppressor | Up | Normal and cancer lung cells | Xi et al. 2010 |
| miR-21 | Fatty acid synthesis, apoptosis | Up | Human squamous carcinoma cells | Zhang et al. 2014 |
| miR-452 | Targets CDK1 | Down | Human alveolar macrophages | Graff et al. 2012 |
Izzotti et al. (2009) analyzed miRNA expression patterns in the lungs of mice exposed to passive cigarette smoke, and they established life-course–related miRNA expression changes by comparing miRNA expression in lungs from unexposed newborn, postweaning, and adult mice. These researchers observed developmental-stage–specific miRNA expression profiles in which miRNAs that were highly expressed in newborns tended to be less expressed in adult mice and vice versa, whereas miRNA expression in postweaning mice was intermediate (Izzotti et al. 2009). Results from in vivo studies concerning smoking and miRNAs are shown in Table 3.
Table 3.
In vivo studies on the effects of smoking on differential miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Source |
|---|---|---|---|---|
| miR-34b | p53 effector | Down | Mouse lung | Izzotti et al. 2011 |
| miR-421 | Targets SMAD4, polycomb gene CBX7, ATM | |||
| miR-450b | No validated targets | |||
| miR-466 | No validated targets | |||
| miR-469 | Mouse miR not validated | |||
| miR-135b | Inflammation, oxidative stress | Up | Mouse lung | Halappanavar et al. 2013 |
| miR-206 | Targets SERP1, BDNF, FOXP1 | Up | Rat serum | Wu et al. 2013 |
| miR-133b | Targets LAG1, PTBP2 | |||
| miR-20b | Hypoxia | Down | Mouse lung and plasma | Huang et al. 2012 |
| miR-30e | Targets UBC9, UBE21, MUC17 | |||
| miR-125b | Targets p53, stress response | |||
| miR-128 | Apoptosis | |||
| let-7a | Cell proliferation, angiogenesis | Down | Mouse lung | Izzotti et al. 2009 |
| let-7b | Cell proliferation, angiogenesis | |||
| let-7f | Cell proliferation, angiogenesis | |||
| miR-26a | Transforming growth factor expression | |||
| miR-30b | Cell adhesion, stress response | |||
| miR-30c | Cell cycle, oncogene activation | |||
| miR-34b | p53 effector | |||
| miR-99b | Apoptosis | |||
| miR-122a | Stress response | |||
| miR-124a | Stress response, cell growth and differentiation | |||
| miR-125a | Oncogene activation, ROS | |||
| miR-125b | Targets p53, stress response | |||
| miR-140 | p53 effector | |||
| miR-192 | Oncogene activation | |||
| miR-431 | Protein repair, oncogene activation | |||
| miR-92b | Tumor suppressomiR | Down | Mouse serum | Yuchuan et al. 2014 |
| miR-668 | Inflammation | |||
| miR-700 | Inflammation | |||
| Let-7e | Apoptosis | Up | Mouse serum | Yuchuan et al. 2014 |
| miR-19a | OncomiR | |||
| miR-142 | Immunology | |||
| miR-191 | OncomiR | |||
| miR-350 | Unknown | |||
| Abbreviations: oncomiR, miR with oncogenic properties; ROS, reactive oxygen species; suppressomiR, tumor suppresor miR. | ||||
Two studies reported a comparison between mRNA and miRNA whole genome expression patterns for smokers and nonsmokers (Schembri et al. 2009; Takahashi et al. 2013). Takahashi et al. (2013) reported that quitting smoking altered the plasma miRNA profiles to resemble those of nonsmokers. In addition, Let-7c and miR-150 could be of importance in the initiation of smoke-induced decline of lung function, because genes that were associated with lung function impairment in genome-wide association studies have been reported to be significantly enriched in binding sites for these miRNAs, namely STAT3 (Qu et al. 2009) and TNFR-II (D’hulst et al. 2006).
The effect of in utero exposures on health during childhood and later in life is a growing area of research interest with major public health implications (Gluckman et al. 2008). An adaptive response in the fetus to in utero exposures can result in persistent changes into adulthood. miRNA expression levels in placenta can affect health later in life (Maccani et al. 2011). Studies on miRNA expression and human exposure at different stages of life (in utero, adult) are included in Table 4.
Table 4.
Human studies on the effects of exposure to smoking on differential miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Source |
|---|---|---|---|---|
| miR-16 | p53, cell cycle, JAK/STAT signaling | Down | Placenta | Maccani et al. 2010 |
| miR-21 | Fatty acid synthesis, apoptosis | |||
| miR-146a | Inflammation, NFκβ mediator | |||
| miR-223 | Immunology | Up | Maternal and cord blood | Herberth et al. 2013 |
| miR-129 | Cell cycle regulation, apoptosis | Down | Spermatozoa | Marczylo et al. 2012 |
| miR-634 | Inflammation | |||
| miR-340 | Cell migration and invasion | Up | Spermatozoa | Marczylo et al. 2012 |
| miR-365 | Targets NKX2.1 | |||
| miR-143 | Cardiogenesis | Down | Gastric tissue | Stánitz et al. 2013 |
| miR-21 | Fatty acid biosynthesis, apoptosis | Up | Gastric tissue | Stánitz et al. 2013 |
| Let-7c | Cell proliferation, angiogenesis | Down | Induced sputum | Van Pottelberge et al. 2011 |
| miR-146a | Inflammation, NFκβ mediator | |||
| miR-150 | Hematopoeiesis | |||
| miR-203 | DNA damage response | |||
| miR-340 | Cell migration and invasion | |||
| miR-443 | Unknown | |||
| miR-223 | Immunology | Down | Plasma MV | Badrnya et al. 2014 |
| miR-29b | Apoptosis | Up | Plasma MV | Badrnya et al. 2014 |
| RNU6-2 | Reference miR | |||
| MV, microvesicles. | ||||
Not surprisingly, miRNAs that are frequently observed to be down-regulated in response to smoking have also been identified as down-regulated in lung (Takamizawa et al. 2004), pancreatic (Vogt et al. 2011), and stomach (Rahman et al. 2009) cancer. Development of cardiovascular disease is associated with up-regulation of miR-206 (Shan et al. 2009), and this miRNA has significantly higher expression levels in smokers than in nonsmokers. Furthermore, two miRNAs that are frequently down-regulated in association with cigarette smoke (i.e., miR-21 and miR-146a) have lower expression levels in individuals with type 2 diabetes compared with healthy controls (Zampetaki et al. 2010). Therefore, these miRNAs could support the observation that smoking is an independent risk factor for type 2 diabetes (Cho et al. 2009). Human studies concerning smoking-induced changes of miRNA expression are summarized in Table 4. Figure 3 is a Venn diagram displaying the common and distinct miRNAs from in vitro, in vivo, and human studies on smoking-induced miRNA alterations. miR-125b and miR-21, identified in in vivo and human studies, respectively, were also reported in in vitro studies. Furthermore, several miRNAs were identified in multiple studies, such as miR-34b and miR-146a.
Figure 3.
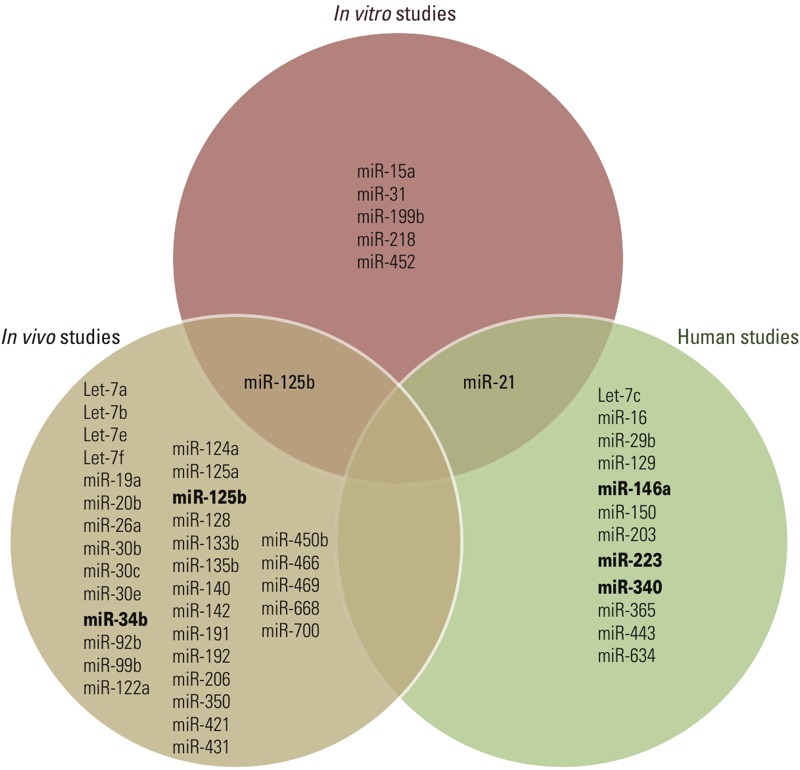
Venn diagram displaying common and distinct microRNAs associated with smoking in in vitro, in vivo, and human studies. miRNAs in bold type were identified in more than one study included in this meta-analysis.
Table 1 summarizes miRNAs with altered expression in response to environmental and/or personal exposures reported in at least two independent studies, along with their known roles in disease etiology. miRNAs observed in association with either environmental or personal exposures are often associated with cancer; in particular, breast and lung cancer and leukemia have been frequently reported (Table 1). Furthermore, many aberrations in the cardiovascular system have been reported, such as hypertension, heart failure, myocardial infarct, and atherosclerosis. Exposures such as air pollution and smoking can cause cardiovascular disease and cancer (Pope et al. 2011); however, the data shown in Table 1 indicate that the listed miRNAs play a causative role in disease etiology, rather than being merely a marker of exposure.
Air pollution exposure and miRNA expression. Particulate matter (PM) is a complex mixture of small particles and liquid droplets. Particle pollution is made up of a number of components, including acids, organic chemicals, metals, and soil or dust particles. The size of particles is directly linked to their potential to cause health problems (Brunekreef and Holgate 2002). Although the clinical effects of PM exposure are obvious, the underlying mechanism of disease initiation and progression is less well understood. miRNAs play a pivotal role in maintaining healthy lungs (Nana-Sinkam et al. 2009). Because the lungs are an important target site for PM, we suggest that miRNAs could underlie the observed health effects of PM exposure. In vitro studies on air pollution and miRNAs are summarized in Table 5.
Table 5.
In vitro studies on air pollution–induced changes in miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Pollutant | Source |
|---|---|---|---|---|---|
| miR-26b | Wnt, p53, autophagy, TGF-β | Down | Primary human bronchial epithelial cells | 10 μg/cm2 DEP | Jardim et al. 2009 |
| miR-27a | Apoptosis, ERα | ||||
| miR-31 | Apoptosis, tumor supressor | ||||
| miR-96 | Several unrelated targets | ||||
| miR-135b | Inflammation, oxidative stress | ||||
| miR-374a | Targets DICER, ATM | ||||
| miR-513c | No validated targets | Up | Primary human bronchial epithelial cells | 10 μg/cm2 DEP | Jardim et al. 2009 |
| miR-513b | No validated targets | ||||
| miR-513a-5p | Targets CD274, immunology | ||||
| miR-923 | Fragment of 28S RNA | ||||
| miR-494 | Targets PTEN | ||||
| miR-338-5p | ABC transporters, endocytosis | ||||
| miR-10b | Angiogenesis | Down | Human A549 lung carcinoma cell line | 1 ppm CH2O | Rager et al. 2011 |
| miR-181a | Apoptosis, oncomiR | ||||
| miR-330 | Targets E2F1, VEGFa, NTRK3 | ||||
| miR-338-5p | ABC transporters, endocytosis | ||||
| miR-375 | Immunology | Up | Human bronchial epithelial cells | 3 μg/cm2 DEP | Bleck et al. 2013 |
| miR-149 | Immunology | Down | Monkey airway epithelial cells | Ozone | Clay et al. 2014 |
| miR-128 | Apoptosis | Up | Human A549 lung carcinoma cell line | PM10 | Motta et al. 2013 |
| Abbreviations: CH2O, formaldehyde; DEP, diesel exhaust particles; OncomiR, miR with oncogenic properties. | |||||
In a cohort study of steel plant workers, Bollati et al. (2010) examined the effect of PM exposure on miRNA expression. Blood samples were collected at the beginning of the working week (“preexposure”) and at the end of the working week (“postexposure”). PM mass and metal components measured in the plant were correlated with miRNA expression analyses of blood samples. Urinary 8-hydroxy-2´-deoxyguanosine (8-OH-dG) levels were measured as a readout of oxidative stress. Both miR-222 and miR-21 were significantly increased in post- versus preexposure samples, and only miR-21 expression levels were positively correlated with 8-OH-dG (Bollati et al. 2010). Oxidative stress has been reported to induce miR-21 expression (Cheng et al. 2009); thus, the association between 8-OH-dG and miR-21 might simply reflect the response of miR-21 to production of reactive oxygen species (ROS) in the blood due to the PM-induced increase in oxidative stress (Bollati et al. 2010) (Table 6).
Table 6.
Human studies on air pollution–induced changes in miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Pollutant | Source |
|---|---|---|---|---|---|
| miR-21 | Fatty acid synthesis, apoptosis | Up | Peripheral blood | 300 μg PM2.5/m3 DEP | Yamamoto et al. 2013 |
| miR-30e | Targets UBC9, MUC17 | ||||
| miR-144 | Targets Klfd, FGG, PLAG1 | ||||
| miR-215 | Cell cycle, p53 activation | ||||
| miR-21 | Fatty acid synthesis, apoptosis | Up | Blood leukocytes | Metal-rich PM | Bollati et al. 2010 |
| miR-222 | Cell cycle regulation | ||||
| miR-375 | Immunology | Up | Bronchial epithelial cells | 3 μg/cm2 DEP | Bleck et al. 2013 |
| miR-34a | Cardiogenesis | Up | Gastric tissue | Urban living | Stánitz et al. 2013 |
| miR-143 | Cardiogenesis | ||||
| miR-10b | Angiogenesis | Up | Spermatozoa | Metal-rich PM | Li et al. 2012a |
| miR-33b | Lipid metabolism | ||||
| miR-106a | OncomiR | ||||
| miR-155 | Inflammation | ||||
| miR-183 | OncomiR | ||||
| miR-205 | OncomiR | ||||
| miR‑208a | Cardiac hypertrophy | ||||
| miR-222 | Cell cycle regulation | ||||
| miR-223 | Immunology | ||||
| Let-7d | Proliferation, angiogenesis | Down | Spermatozoa | Metal-rich PM | Li et al. 2012a |
| miR-363 | DNA damage response | ||||
| miR-25 | DNA damage response | Up | Induced sputum | Ozone | Fry et al. 2014 |
| miR-132 | Angiogenesis | ||||
| miR-143 | Cardiogenesis | ||||
| miR-145 | Tumor suppressor | ||||
| miR-199a | Oncogene activation | ||||
| miR-199b | Oncogene activation | ||||
| miR-222 | Cell cycle regulation | ||||
| miR-223 | Immunology | ||||
| miR-424 | Angiogenesis | ||||
| miR-582 | Antiapoptosis | ||||
| miR-1 | Apoptosis | Down | Leukocytes | PM2.5, black carbon, organic carbon, sulfate | Fossati et al. 2014 |
| miR-9 | Neuronal differentiation | ||||
| miR-21 | Fatty acid synthesis, apoptosis | ||||
| miR-126 | Angiogenesis | ||||
| miR-135a | Inflammation | ||||
| miR-146a | Inflammation, NFκβ mediator | ||||
| miR-155 | Inflammation | ||||
| miR-222 | Cell cycle regulation | ||||
| miR-128 | Apoptosis | Up | Plasma MV | PM10 | Motta et al. 2013 |
| Abbreviations: DEP, diesel exhaust particles; MV, microvesicles; OncomiR, miR with oncogenic properties; PM2.5, particulate matter ≤ 2.5 μm in aerodynamic diameter. | |||||
The cardiovascular anomalies observed in association with air pollution exposure have often been attributed to the generation of oxidative stress (Miller et al. 2012). MiR-21 is up-regulated in response to diesel exhaust particles and metal-rich PM (Bollati et al. 2010; Bourdon et al. 2012) and is highly expressed in the cardiovascular system, where it plays an important role in vascular cell proliferation and apoptosis and disease [reviewed by Cheng and Zhang (2010)]. Therefore, miR-21 expression could be an important mechanistic link explaining the association between air pollution exposure and cardiovascular disease.
Levänen et al. (2013) observed distinct miRNA expression profiles in patients with asthma compared with controls after subway exposure. Current epidemiological studies have identified the first miRNAs associated with air pollution exposure, and provide a list of putative biomarkers. Table 6 summarizes the human studies on air pollution and miRNAs. A Venn diagram displays the common and distinct miRNAs from in vitro and human studies on air pollution–induced miRNA alterations (Figure 4). The only miRNAs identified in both in vitro and human studies in association with air pollution exposure are miR-10b and miR-128. Furthermore, miRNAs -9, -21, -143, -155, -222, -223, and -338 were identified in at least two independent studies on air pollution and miRNA.
Figure 4.
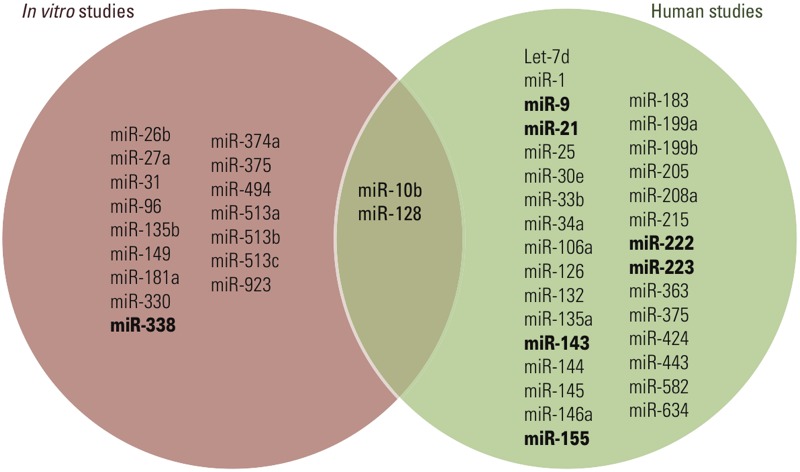
Venn diagram displaying common and distinct microRNAs associated with air pollution exposure in in vitro and human studies. miRNAs in bold type were identified in more than one study included in this meta-analysis.
Nanoparticles. Nanoparticles are emitted from natural and anthropogenic sources and are produced via nanotechnology. Fast propagation of nanotechnologies into different industries and consumer products is causing exponential growth of nanomaterial production. Hence, increasing amounts of nanoparticles reach occupational settings and the indoor and outdoor environments, thus representing a potentially serious hazard to human health (Castranova 2011; Nel et al. 2006). Nanoparticles are also able to cross cell membranes, and their interactions with biological systems are relatively unknown (Holsapple et al. 2005). Table 7 includes the studies on nanoparticle-induced changes in miRNA expression, all of which were performed in animal models.
Table 7.
Studies on nanoparticle-induced changes in miRNA expression.
| miRNA | miR function | Regulation | Pollutant | Source |
|---|---|---|---|---|
| miR-21 | Fatty acid synthesis, apoptosis | Up | 0.268 or 0.162 mg carbon black NP | Bourdon et al. 2012 |
| miR-135b | Inflammation, oxidative stress | |||
| miR-146 | Inflammation, NFκβ mediator | |||
| miR-122 | Stress response | Up | 70 nm in silica NP | Nagano et al. 2013 |
| miR-192 | Oncogene activation | |||
| Let-7a | Cell proliferation, angiogenesis | Up | 100 nm gold NP | Balansky et al. 2013 |
| miR-183 | OncomiR | |||
| Abbreviations: NP, nanoparticles; oncomiR, miR with oncogenic properties. | ||||
Chemical exposure-induced changes in miRNA. Formaldehyde. Formaldehyde is an air toxic present in the atmosphere due to emission from anthropogenic and biogenic sources. Ninety-five percent of inhaled formaldehyde is absorbed within the respiratory tract (Overton et al. 2001). Formaldehyde has been reported to change gene expression patterns in nasal and lung cells (Kim et al. 2002; Li et al. 2007). The miRNAs reported to be down-regulated in association with formaldehyde exposure have been reported to be involved in the development of diverse tumors (e.g., breast and gastrointestinal cancer, melanoma) as well as heart failure (Table 1). Given the capability of formaldehyde to pass deep into lung tissue and enter systemic circulation, the link with cardiovascular disease and cancer has been widely discussed [reviewed by Kim KH et al. (2011)]. Interestingly, miR-181a, one of the miRNAs down-regulated after formaldehyde exposure, was reported to affect the DNA damage response in breast cancer, enabling the identification of aggressive breast tumors based on increased miR-181a expression (Bisso et al. 2013).
Endocrine disruptors. Organochlorine pesticides and plasticizing agents are ubiquitous environmental endocrine-disrupting compounds that impact human health (Rubin 2011). Bisphenol A (BPA) is an industrial plasticizer often used as a coating in food cans and in plastic bottles (Kang et al. 2006). Dichlorodiphenyltrichloroethane (DDT) is a well-known organochlorine pesticide. Because DDT is very persistent in the environment, accumulates in fatty tissues, and can travel long distances in the upper atmosphere, residues from historical use remain a current threat to human health.
DDT and BPA have been reported to interfere with endogenous estrogens and thyroid hormone, leading to aberrations of the reproductive, immune, and central nervous systems (Chevrier et al. 2013; Liu et al. 2013). DDT (Waliszewski et al. 2001) and BPA (Takahashi and Oishi 2000) cross the placental barrier and can induce in utero effects that could lead to detrimental effects later in life.
Soto et al. (2013) reported that prenatal exposure to BPA can alter mammary development and lead to breast cancer in humans. From a clinical perspective, it is interesting that decreased expression of let-7f has been associated with increased breast cancer risk (Sakurai et al. 2012), and treatment of MCF-7 breast cancer cells with BPA resulted in reduced let-7f expression (Tilghman et al. 2012). Furthermore, miR-146a has been proposed to induce an Alzheimer’s disease pathway (Jiang et al. 2013) and is up-regulated after BPA exposure (Table 1). Therefore, the neurodegenerative consequences of BPA exposure could at least partially be attributed to miR-146a. In vitro studies could provide researchers with interesting miRNAs that have potential to be used as biomarkers for chemical exposure.
Polychlorinated biphenyls (PCBs) were widely used organic chemicals until their production was banned because of environmental concerns (Porta and Zumeta 2002). PCBs are stable compounds that bioaccumulate in fatty tissues (Steele et al. 1986); they have been reported to cause systemic changes in gene expression (Ceccatelli et al. 2006), suggesting that miRNA regulation could be involved in this process. Tsukimori et al. (2008) reported an association between maternal PCB exposure and fetal toxicity, impaired fetal growth, and pregnancy loss.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) has been reported to adversely affect the immune system in rats (Faith and Luster 1979). In addition, Camacho et al. (2004) reported that TCDD exposure of pregnant mice affected the immune system of fetuses by suppressing T-cell function. Given the regulatory role miRNAs play in the immune system (Contreras and Rao 2012), it can be expected that miRNAs are important in regulating the detrimental health effects observed after exposure to TCDD and PCBs.
Arsenic. Environmental exposure to arsenic, especially to trivalent inorganic arsenic (As3+), is a health concern given the high concentrations present in groundwater across the world (Fendorf et al. 2010). Exposure to arsenic has been associated with increased risk of cancer due to genomic instability (Dulout et al. 1996), and long-term arsenic exposure has been observed to induce peripheral vascular injury (Tseng 2008). A Venn diagram showing the common and distinct miRNAs from in vitro and human studies on arsenic-induced miRNA alterations is presented in Figure 5. Only miRNA-21 was associated with arsenic exposure in in vitro model systems and in human studies. Three miRNAs were identified by at least two independent studies on arsenic exposure and miRNA expression, namely, miR-26b, miR-181a, and miR-222.
Figure 5.
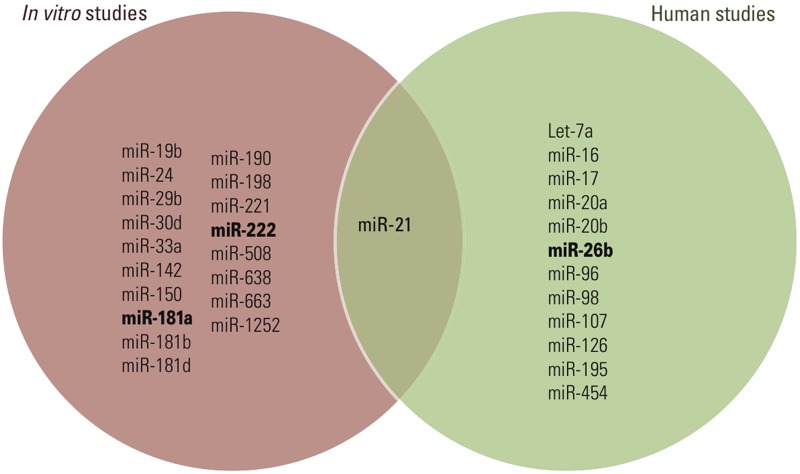
Venn diagram displaying common and distinct microRNAs associated with arsenic exposure in in vitro and human studies. miRNAs in bold type were identified in more than one study included in this meta-analysis.
Aluminum sulfates. Aluminum is the most widely distributed metal in the environment and is extensively used in daily life. Chronic exposure of animals to aluminum is associated with behavioral and neuropathological changes (Fulgenzi et al. 2014). Epidemiological studies have shown poor performance in cognitive tests and a higher abundance of neurological symptoms in workers occupationally exposed to aluminum (Kumar and Gill 2009).
Hexahydro-1,3,5-trinitro-s-triazine (RDX). The polynitramine explosive RDX is a heavily used second-generation high explosive, and its use can result in the contamination of soils, sediments, and water (Davis et al. 2004). RDX exposure has been reported to be toxic to the neural and immune systems and to increase tumor incidence in several cancers (Garcia-Reyero et al. 2011; Sweeney et al. 2012).
Diethylstilbestrol (DES). The synthetic estrogen DES was prescribed to pregnant women from the 1940s to the 1960s in order to prevent miscarriages; however, DES was later reported to be responsible for increasing breast cancer in the mothers and gynecologic tumor incidence in their exposed daughters (Greenberg et al. 1984; Mittendorf 1995).
Perfluorooctanoic acid (PFOA). Perfluoroalkyl chemicals (PFCs) are highly stable and widely used in industrialized countries. PFCs are both lipophobic and hydrophobic; thus, after absorption they will bind to proteins in serum and liver rather than accumulate in lipids. PFOA is one of the most commonly used PFCs.
The studies we reviewed on chemical-induced changes in miRNA expression are summarized in Tables 8–10 by type of study: in vitro (Table 8), in vivo (Table 9), and human (Table 10) studies.
Table 8.
In vitro studies on chemically induced changes in miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Chemical | Source |
|---|---|---|---|---|---|
| let-7g | Cell proliferation, angiogenesis | Down | MCF-7 cells | BPA | Tilghman et al. 2012 |
| let-7f | Cell proliferation, angiogenesis | ||||
| miR-21 | Fatty acid biosynthesis, apoptosis | ||||
| miR-26b | Wnt, p53, autophagy, TGF-β | ||||
| miR-342-3p | Tumor suppressomiR | ||||
| miR-15b | Tumor suppressor targeting BCL2 | Down | MCF-7 cells | BPA, DDT | Tilghman et al. 2012 |
| miR-222 | Cell cycle regulation | Up | MCF-7 cells | BPA | Tilghman et al. 2012 |
| miR-638 | No known function | Up | MCF-7 cells | BPA, DDT | Tilghman et al. 2012 |
| miR-663 | Immunology, oxidative stress | Down | MCF-7 cells | DDT | Tilghman et al. 2012 |
| miR-1915 | No known function | ||||
| miR-27b | Angiogenesis | ||||
| miR-92a | Tumor supressomiR | ||||
| miR-92b | Tumor supressomiR | ||||
| miR-1308 | No known function | Up | MCF-7 cells | DDT | Tilghman et al. 2012 |
| miR-146a | Inflammation, NFκβ mediator | Up | Human placental cell lines | BPA | Avissar-Whiting et al. 2010 |
| miR-150 | Hematopoeiesis | Down | Jurkat T cell line | Arsenic | Sturchio et al. 2014 |
| miR-30d | Autophagy | Up | Jurkat T cell line | Arsenic | Sturchio et al. 2014 |
| miR-142 | Immunology | ||||
| miR-181a | Apoptosis, oncomiR | ||||
| miR-221 | DNA damage response | ||||
| miR-222 | Cell cycle regulation | ||||
| miR-638 | No known function | ||||
| miR-663 | Immunology, oxidative stress | ||||
| miR-190 | OncomiR | Up | Human bronchial epithelial cells | Arsenic | Beezhold et al. 2011 |
| miR-19b | OncomiR | Up | HUVEC cells | Arsenic | Li et al. 2012b |
| miR-21 | Fatty acid biosynthesis, apoptosis | ||||
| miR-24 | OncomiR | ||||
| miR-29b | Apoptosis | ||||
| miR-33a | Lipid metabolism | ||||
| miR-198 | Cell proliferation | ||||
| miR-508-5p | Cell invasion and migration | ||||
| miR-1252 | No known function | ||||
| miR-181a | Apoptosis, oncomiR | Up | HepG2 cells | PAH | Song et al. 2013 |
| miR-181b | Apoptosis, oncomiR | ||||
| miR-181d | Apoptosis, oncomiR | ||||
| Abbreviations: BPA, bisphenol A; DDT, dichlorodiphenyltrichloroethane; OncomiR, miR with oncogenic properties; PAH, polycyclic aromatic hydrocarbon; tumor suppressomiR, tumor suppressor miR. | |||||
Table 10.
Human studies on chemically induced changes in miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Chemical | Source |
|---|---|---|---|---|---|
| miR-191 | OncomiR | Up | Peripheral blood | PCB-169 | Guida et al. 2013 |
| miR-146a | Inflammation, NFκβ mediator | Up | Fetal brain cells | Aluminum | Pogue et al. 2009 |
| miR-9 | Neuronal differentiation | ||||
| miR-125b | Targets p53, stress response | Up | Fetal brain cells | Aluminum | Lukiw and Pogue 2007 |
| miR-128 | Apoptosis | ||||
| miR-199a | Oncogene activation | Up | Serum | PFOA | Wang J et al. 2012 |
| miR-21 | Fatty acid biosynthesis, apoptosis | Up | Blood samples | Arsenic | Kong et al. 2012 |
| miR-26b | Wnt, p53, autophagy, TGF-β | ||||
| Let-7a | Cell proliferation, angiogenesis | Up | Cord blood | Arsenic | Rager et al. 2014 |
| miR-16 | p53, cell cycle, JAK/STAT | ||||
| miR-17 | DNA damage response | ||||
| miR-20a | Angiogenesis | ||||
| miR-20b | Hypoxia | ||||
| miR-26b | Wnt, p53, autophagy, TGF-β | ||||
| miR-96 | Several unrelated targets | ||||
| miR-98 | Apoptosis | ||||
| miR-107 | Targets Notch2 | ||||
| miR-126 | Angiogenesis | ||||
| miR-195 | Tumor suppressomiR | ||||
| miR-454 | Unknown | ||||
| miR-24 | OncomiR | Down | Plasma | PAH | Deng et al. 2014 |
| miR-27a | Apoptosis, ERα | ||||
| miR-28 | Apoptosis | ||||
| miR-142 | Immunology | ||||
| miR-150 | Hematopoeiesis | Up | Plasma | PAH | Deng et al. 2014 |
| Abbreviations: OncomiR, miR with oncogenic properties; PAH, polycyclic aromatic hydrocarbon; suppressomiR, tumor suppressor miR. | |||||
Table 9.
In vivo studies on chemically induced changes in miRNA expression.
| miRNA | miR function | Regulation | Tissue/cell type | Chemical | Source |
|---|---|---|---|---|---|
| let-7e | Apoptosis | Down | Fetal mouse thymocytes | TCDD | Singh et al. 2012 |
| miR-18b | Apoptosis | ||||
| miR-23a | Apoptosis | ||||
| miR-23b | Apoptosis | ||||
| miR-27a | Apoptosis, ERα | ||||
| miR-28 | Apoptosis | ||||
| miR-29a | Apoptosis | ||||
| miR-31 | Apoptosis, tumor supressomiR | ||||
| miR-98 | Apoptosis | ||||
| miR-101b | Apoptosis | ||||
| miR-181c | Apoptosis, oncomiR | ||||
| miR-182 | Apoptosis | ||||
| miR-200a | Apoptosis, cell cycle, MAPK | ||||
| miR-23 | Apoptosis | ||||
| miR-290 | Apoptosis | ||||
| miR-335 | Apoptosis | ||||
| miR-491 | Apoptosis, targets BCL-XL | ||||
| miR-122 | Stress response | Up | Fetal mouse thymocytes | TCDD | Singh et al. 2012 |
| miR-181a | OncomiR | ||||
| miR-125b | Targets p53, stress response | Up | Monkey nasal epithelium | Formaldehyde | Rager et al. 2013 |
| miR-152 | Tumor suppressor, methylation | ||||
| miR-219 | NMDA receptor signaling | ||||
| miR-532 | Unknown | ||||
| miR-22 | PTEN, AKT signaling | Down | Monkey nasal epithelium | Formaldehyde | Rager et al. 2013 |
| miR-26b | Wnt, p53, autophagy, TGF-β | ||||
| miR-29a | Apoptosis | ||||
| miR-140 | p53 effector | ||||
| miR-142 | Immunology | ||||
| miR-145 | Tumor suppressor, stem cell different | ||||
| miR-203 | DNA damage response | ||||
| miR-374a | Targets DICER, ATM | ||||
| miR-520f | Unknown | ||||
| miR-27a | Apoptosis, ERα | Down | Mouse brain and liver | RDX | Zhang and Pan 2009 |
| miR-200c | Apoptosis | ||||
| let7-e | Apoptosis | ||||
| miR-206 | Targets SERP1, BDNF, FOXP1 | ||||
| miR-451 | Targets PI3K/AKT | Down | Rat liver | PFOS | Wang et al. 2014 |
| miR-23a | Apoptosis | Up | Rat liver | PFOS | Wang et al. 2014 |
| miR-25 | DNA damage response | ||||
| miR-125a | Oncogene activation, ROS | ||||
| miR-133a | Smooth muscle differentiation | ||||
| miR-133b | Targets LAG1 and PTBP2 | ||||
| miR-206 | Targets SERP1, BDNF, FOXP1 | ||||
| miR-494 | Targets PTEN | ||||
| miR-542 | DNA damage response | ||||
| Abbreviations: OncomiR, miR with oncogenic properties; PFOS, perfluorooctane sulfonate; RDX, hexahydro-1,3,5-trinitro-s-triazine; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin. | |||||
Conclusions
miRNAs are omnipresent in the genome and are important regulators of gene expression in response to intracellular as well as environmental cues. In this review, we examined the response of the miRNA machinery to personal and environmental exposures, including air pollution, cigarette smoking, and chemicals such as endocrine disruptors. miRNAs have been proposed as biomarkers for disease; however, the literature also reveals their potential to be used as biomarkers of environmental exposure.
In different studies on the same environmental pollutant, overall the identified miRNAs showed similar patterns of expression regulation. In studies where smoking-induced changes were investigated, the general observation was a down-regulation of expression. For example, miR-125b was down-regulated in response to cigarette smoke in both primary human bronchial epithelial cells (Schembri et al. 2009) and mouse lung tissue (Izzotti et al. 2009). However, when unique miRNAs had altered expression patterns in response to different environmental exposures, their direction of regulation could be the same (10/25 miRNAs) or the opposite (15/25 miRNAs; 60%). The different exposures we discussed here have their own unique health effects, so one would not expect them to have the same effect on the miRNA machinery. However, there is sometimes a discrepancy when looking at the same exposure indicator; for example, in response to smoking, miR-21 has been reported to be up-regulated in some studies and down-regulated in others (Table 4). Part of the discrepancy can be explained by the different exposure models that were used.
In general, different in vitro studies show little overlap, potentially because of the complex miRNA–mRNA networks that underlie the observations and the differences in exposure used across studies. In studies of the same environmental pollutant in vitro, in vivo, or in humans, identified miRNAs were quite distinct (Figures 3–5). This can be explained in part by the observation that animal models do not always reflect genomic responses that occur in humans (Seok et al. 2013). Discrepancy between different studies might also stem from differences in exposure duration. For example, in a study in rats, the duration of exposure uniquely influenced expression patterns of the individual miRNAs (Izzotti et al. 2011).
Human epidemiological studies are necessary to observe exposure-related effects on miRNAs. Understanding the exposome requires putting together pieces of a complex puzzle. Epidemiological studies need input from experimental studies to identify good candidate biomarkers, and results from epidemiological studies often need follow-up by experimental studies to investigate mechanisms of action and to study tissue dependency of effects because human studies are most often performed in easily accessible tissues such as blood and saliva as a surrogate for the actual target tissues.
Currently, epidemiological studies on microRNA often involve free or exosomal miRNAs present in saliva or other body fluids. However, it is not clear whether these observed miRNA changes are a true reflection of the body’s response and can really predict health effects. In blood, miRNAs within the exosomes have been shown to overlap with cellular miRNA profiles: Cheng et al. (2014) observed that exosomes derived from blood were enriched for miRNAs and that miRNA profiles between blood cells and the cell-free exosomal fraction showed important overlap.
Because miRNAs can regulate mRNA expression in both a negative manner and a positive manner (Vasudevan et al. 2007) and because many miRNAs can bind the same mRNA (Saetrom et al. 2007), it is difficult to draw conclusions from miRNA studies without infomation on the concurrent mRNA(s) expression pattern. However, this information is rare in current reports on epidemiological studies of miRNAs. The findings of this review underscore the complex networks that are built by miRNAs and the mRNAs they regulate because one miRNA can influence many mRNAs according to the timing and pattern of expression.
Many of the reviewed studies used large-scale microarray profiling, but follow-up and validation with more quantitative approaches often lags behind. This delay is understandable because of the cost and labor intensity inherent to these techniques; however, it is important to confirm the miRNAs that are responsive to environmental exposures.
Researchers are currently publishing extensive lists of miRNAs that are responsive to environmental exposures and showing their utility as biomarkers of effect. Future research should focus on identifying the molecular mechanism behind miRNA expression changes in response to exposure to determine whether the changes in miRNA expression are merely a symptom of the (patho)physiological processes the organism undergoes after exposure, or whether miRNAs are the drivers responsible for these changes. Izzotti and Pulliero (2014) recently reviewed the putative mechanisms of action behind miRNAs’ response to environmental exposure. However, the effect of the identified miRNAs on putative mRNA targets should also be studied to determine whether the change in miRNA expression has functional consequences and which mRNAs are true miRNA targets under the given circumstances.
At present, little is known about whether environmental exposures induce long-term changes in human miRNA expression or whether these have a transient character. To address this problem, more longitudinal studies should be conducted to examine the long-term effects of exposure. Results from animal studies suggest that miRNA expression changes in response to formaldehyde exposure are transient and revert to normal levels after recovery from exposure (Rager et al. 2014), but Izzotti et al. (2011) reported that miRNA profiles in target organs did not recover 1 week after cessation of long-term cigarette smoke exposure. In a study in humans, Takahashi et al. (2013) observed that miRNA expression profiles of individuals who quit smoking resembled those of nonsmokers.
Follow-up in future generations is necessary to determine the heritability of the miRNA expression changes. It would be very interesting to examine the effect of in utero environmental exposures on development of disease in later life and the role miRNAs play in inducing these health effects. Furthermore, long-term longitudinal studies would allow us to distinguish between cause and effect of miRNA expression and environmental exposure, and would also allow us to estimate the contribution of miRNAs to disease development. Studies have shown that miRNAs can be used as biomarkers of disease as well as biomarkers for environmental exposure and that miRNAs hold great potential to explain disease etiology.
Acknowledgments
We thank emeritus professor H.A. Roels (Université catholique de Louvain, Brussels, Belgium) for critical discussions and reading of the manuscript.
Footnotes
K.V. is a postdoctoral researcher of the Research Foundation–Flanders (FWO). V.B. and T.S.N. received support from the European Union Programme “Ideas” (ERC-2011-StG 282413 and ERC-2012-StG 310898).
The funders had no role in study design, data collection, decision to publish, or preparation of the manuscript.
The authors declare they have no actual or potential competing financial interests.
References
- Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, et al. A uniform system for microRNA annotation. RNA. 2003;9(3):277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar-Whiting M, Veiga KR, Uhl KM, Maccani MA, Gagne LA, Moen EL, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;29(4):401–406. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrnya S, Baumgartner R, Assinger A. Smoking alters circulating plasma microvesicle pattern and microRNA signatures. Thromb Haemost. 2014;112(1):128–136. doi: 10.1160/TH13-11-0977. [DOI] [PubMed] [Google Scholar]
- Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284(46):32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balansky R, Longobardi M, Ganchev G, Iltcheva M, Nedyalkov N, Atanasov P, et al. Transplacental clastogenic and epigenetic effects of gold nanoparticles in mice. Mutat Res. 2013;751–752:42–48. doi: 10.1016/j.mrfmmm.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Beezhold K, Liu J, Kan H, Meighan T, Castranova V, Shi X, et al. miR-190-mediated downregulation of PHLPP contributes to arsenic-induced Akt activation and carcinogenesis. Toxicol Sci. 2011;123(2):411–420. doi: 10.1093/toxsci/kfr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisso A, Faleschini M, Zampa F, Capaci V, de Santa J, Santarpia L, et al. Oncogenic miR-181a/b affect the DNA damage response in aggressive breast cancer. Cell Cycle. 2013;12(11):1679–1687. doi: 10.4161/cc.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleck B, Grunig G, Chiu A, Liu M, Gordon T, Kazeros A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol. 2013;190(7):3757–3763. doi: 10.4049/jimmunol.1201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati V, Marinelli B, Apostoli P, Bonzini M, Nordio F, Hoxha M, et al. 2010Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect 118763–768.; 10.1289/ehp.0901300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon JA, Saber AT, Halappanavar S, Jackson PA, Wu D, Hougaard KS, et al. Carbon black nanoparticle intratracheal installation results in large and sustained changes in the expression of miR-135b in mouse lung. Environ Mol Mutagen. 2012;53(6):462–468. doi: 10.1002/em.21706. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Busk PK, Cirera S. MicroRNA profiling in early hypertrophic growth of the left ventricle in rats. Biochem Biophys Res Commun. 2010;396(4):989–993. doi: 10.1016/j.bbrc.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353(17):1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on maternal immune response during pregnancy. Arch Toxicol. 2004;78(5):290–300. doi: 10.1007/s00204-003-0538-8. [DOI] [PubMed] [Google Scholar]
- Caramuta S, Egyházi S, Rodolfo M, Witten D, Hansson J, Larsson C, et al. MicroRNA expression profiles associated with mutational status and survival in malignant melanoma. J Invest Dermatol. 2010;130(8):2062–2070. doi: 10.1038/jid.2010.63. [DOI] [PubMed] [Google Scholar]
- Castranova V. Overview of current toxicological knowledge of engineered nanoparticles. J Occup Environ Med. 2011;53(6 suppl):S14–S17. doi: 10.1097/JOM.0b013e31821b1e5a. [DOI] [PubMed] [Google Scholar]
- Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220(2–3):104–116. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Chang S, Wang RH, Akagi K, Kim KA, Martin BK, Cavallone L, et al. Tumor suppressor BRCA1 epigenetically controls oncogenic microRNA-155. Nat Med. 2011;17(10):1275–1282. doi: 10.1038/nm.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sharples RA, Scicluna BJ, Hill AF.2014Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 323743; 10.3402/jev.v3.23743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47(1):5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3(3):251–255. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, et al. 2013Maternal urinary bisphenol A during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect 121138–144.; 10.1289/ehp.1205092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Chan JC, Jang HC, Lim S, Kim HL, Choi SH. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin Endocrinol (Oxf) 2009;71(5):679–685. doi: 10.1111/j.1365-2265.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- Clay CC, Maniar-Hew K, Gerriets JE, Wang TT, Postlethwait EM, Evans MJ, et al. 2014Early life ozone exposure results in dysregulated innate immune function and altered microRNA expression in airway epithelium. PLoS One 93e90401; 10.1371/journal.pone.0090401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J, Rao DS. MicroRNAs in inflammation and immune responses. Leukemia. 2012;26(3):404–413. doi: 10.1038/leu.2011.356. [DOI] [PubMed] [Google Scholar]
- Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009;29(7):749–754. doi: 10.1007/s00296-008-0758-6. [DOI] [PubMed] [Google Scholar]
- Davis JL, Wani AH, O’Neal BR, Hansen LD. RDX biodegradation column study: comparison of electron donors for biologically induced reductive transformation in groundwater. J Hazard Mater. 2004;112(1–2):45–54. doi: 10.1016/j.jhazmat.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Deng Q, Huang S, Zhang X, Zhang W, Feng J, Wang T, et al. 2014Plasma microRNA expression and micronuclei frequency in workers exposed to polycyclic aromatic hydrocarbons. Environ Health Perspect 122719–725.; 10.1289/ehp.1307080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’hulst AI, Bracke KR, Maes T, De Bleecker JL, Pauwels RA, Joos GF, et al. Role of tumour necrosis factor-α receptor P75 in cigarette smoke–induced pulmonary inflammation and emphysema. Eur Respir J. 2006;28:102–112. doi: 10.1183/09031936.06.00059305. [DOI] [PubMed] [Google Scholar]
- Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331(6017):550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulout FN, Grillo CA, Seoane AI, Maderna CR, Nilsson R, Vahter M, et al. Chromosomal aberrations in peripheral blood lymphocytes from native Andean women and children from northwestern Argentina exposed to arsenic in drinking water. Mutat Res. 1996;370(3–4):151–158. doi: 10.1016/s0165-1218(96)00060-2. [DOI] [PubMed] [Google Scholar]
- Earle JS, Luthra R, Romans A, Abraham R, Ensor J, Yao H, et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. J Mol Diagn. 2010;12(4):433–440. doi: 10.2353/jmoldx.2010.090154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717(1–2):85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyholzer M, Schmid S, Schardt JA, Haefliger S, Mueller BU, Pabst T. Complexity of miR-223 regulation by CEBPA in human AML. Leuk Res. 2010;34(5):672–676. doi: 10.1016/j.leukres.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Faith RE, Luster MI. Investigations on the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on parameters of various immune functions. Ann NY Acad Sci. 1979;320:564–571. doi: 10.1111/j.1749-6632.1979.tb56634.x. [DOI] [PubMed] [Google Scholar]
- Feitelson MA, Lee J. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007;252(2):157–170. doi: 10.1016/j.canlet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Fendorf S, Michael HA, van Geen A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science. 2010;328(5982):1123–1127. doi: 10.1126/science.1172974. [DOI] [PubMed] [Google Scholar]
- Ferretti E, De Smaele E, Po A, Di Marcotullio L, Tosi E, Espinola MS, et al. MicroRNA profiling in human medulloblastoma. Int J Cancer. 2009;124(3):568–577. doi: 10.1002/ijc.23948. [DOI] [PubMed] [Google Scholar]
- Fossati S, Baccarelli A, Zanobetti A, Hoxha M, Vokonas PS, Wright RO, et al. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology. 2014;25(1):68–78. doi: 10.1097/EDE.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry RC, Rager JE, Bauer R, Sebastian E, Peden DB, Jaspers I, et al. Air toxics and epigenetic effects: ozone altered microRNAs in the sputum of human subjects. Am J Physiol Lung Cell Mol Physiol. 2014;306(12):L1129–L1137. doi: 10.1152/ajplung.00348.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgenzi A, Vietti D, Ferrero ME.2014Aluminium involvement in neurotoxicity. Biomed Res Int 758323; 10.1155/2014/758323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella S, Rinaldi F, Lepore SM, Viola A, Loro E, Angelini C, et al. 2010Overexpression of microRNA-206 in the skeletal muscle from myotonic dystrophy type 1 patients. J Transl Med 848; 10.1186/1479-5876-8-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, et al. 2012Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis 1155; 10.1186/1476-511X-11-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Yu Y, Cao H, Shen H, Li X, Pan S, et al. Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother. 2010;64(6):399–408. doi: 10.1016/j.biopha.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Garcia-Reyero N, Habib T, Pirooznia M, Gust KA, Gong P, Warner C, et al. Conserved toxic responses across divergent phylogenetic lineages: a meta-analysis of the neurotoxic effects of RDX among multiple species using toxicogenomics. Ecotoxicology. 2011;20(3):580–594. doi: 10.1007/s10646-011-0623-3. [DOI] [PubMed] [Google Scholar]
- Genovesi LA, Carter KW, Gottardo NG, Giles KM, Dallas PB.2011Integrated analysis of miRNA and mRNA expression in childhood medulloblastoma compared with neural stem cells. PloS one 69e23935; 10.1371/journal.pone.0023935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JW, Powers LS, Dickson AM, Kim J, Reisetter AC, Hassan IH, et al. 2012Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PloS One 78e44066; 10.1371/journal.pone.0044066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco S, Fasanaro P, Castelvecchio S, D’Alessandra Y, Arcelli D, Di Donato M, et al. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes. 2012;61(6):1633–1641. doi: 10.2337/db11-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ER, Barnes AB, Resseguie L, Barrett JA, Burnside S, Lanza LL, et al. Breast cancer in mothers given diethylstilbestrol in pregnancy. N Engl J Med. 1984;311(22):1393–1398. doi: 10.1056/NEJM198411293112201. [DOI] [PubMed] [Google Scholar]
- Guida M, Marra M, Zullo F, Guida M, Trifuoggi M, Biffali E, et al. Association between exposure to dioxin-like polychlorinated biphenyls and miR-191 expression in human peripheral blood mononuclear cells. Mutat Res. 2013;753(1):36–41. doi: 10.1016/j.mrgentox.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Halappanavar S, Nikota J, Wu D, Williams A, Yauk CL, Stampfli M. IL-1 receptor regulates microRNA-135b expression in a negative feedback mechanism during cigarette smoke-induced inflammation. J Immunol. 2013;190(7):3679–3686. doi: 10.4049/jimmunol.1202456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JI, Goergen D, Zheng J, Song Y, Schüttler CG, Fehr C, et al. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27(24):3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberth G, Bauer M, Gasch M, Hinz D, Röder S, Olek S, et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol. 2013;133(2):543–550. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Farland WH, Landry TD, Monteiro-Riviere NA, Carter JM, Walker NJ, et al. Research strategies for safety evaluation of nanomaterials, part II: toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicol Sci. 2005;88(1):12–17. doi: 10.1093/toxsci/kfi293. [DOI] [PubMed] [Google Scholar]
- Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med. 2009;47(8):923–929. doi: 10.1515/CCLM.2009.228. [DOI] [PubMed] [Google Scholar]
- Huang Y, Dai Y, Zhang J, Wang C, Li D, Cheng J, et al. Circulating microRNAs as potential biomarkers for smoking-related interstitial fibrosis. Biomarkers. 2012;17(5):435–440. doi: 10.3109/1354750X.2012.680611. [DOI] [PubMed] [Google Scholar]
- Hulsmans M, Sinnaeve P, Van der Schueren B, Mathieu C, Janssens S, Holvoet P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J Clin Endocrinol Metab. 2012;97(7):E1213–E1218. doi: 10.1210/jc.2012-1008. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Calin GA, Steele VE, Croce CM, De Flora S. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J. 2009;23(9):3243–3250. doi: 10.1096/fj.09-135251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Larghero P, Longobardi M, Cartiglia C, Camoirano A, Steele VE, et al. Dose-responsiveness and persistence of microRNA expression alterations induced by cigarette smoke in mouse lung. Mutat Res. 2011;717(1–2):9–16. doi: 10.1016/j.mrfmmm.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Pulliero A. The effects of environmental chemical carcinogens on the microRNA machinery. Int J Hyg Environ Health. 2014;217(6):601–627. doi: 10.1016/j.ijheh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Jardim MJ, Fry RC, Jaspers I, Dailey L, Diaz-Sanchez D.2009Disruption of microRNA expression in human airway cells by diesel exhaust particles is linked to tumorigenesis-associated pathways. Environ Health Perspect 1171745–1751.; 10.1289/ehp.0900756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100(11):1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Meng F, Lian B, Chen X, Yu X, et al. Identification of active transcription factor and miRNA regulatory pathways in Alzheimer’s disease. Bioinformatics. 2013;29(20):2596–2602. doi: 10.1093/bioinformatics/btt423. [DOI] [PubMed] [Google Scholar]
- Jin L, Hu WL, Jiang CC, Wang JX, Han CC, Chu P, et al. MicroRNA-149*, a p53-responsive microRNA, functions as an oncogenic regulator in human melanoma. Proc Natl Acad Sci USA. 2011;108(38):15840–15845. doi: 10.1073/pnas.1019312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju X, Li D, Shi Q, Hou H, Sun N, Shen B. Differential microRNA expression in childhood B-cell precursor acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2009;26(1):1–10. doi: 10.1080/08880010802378338. [DOI] [PubMed] [Google Scholar]
- Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226(2–3):79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee HC, Park JL, Kim M, Kim SY, Noh SM, et al. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics. 2011;6(6):740–751. doi: 10.4161/epi.6.6.15874. [DOI] [PubMed] [Google Scholar]
- Kim KH, Jahan SA, Kabir E. A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192(2):425–431. doi: 10.1016/j.jhazmat.2011.05.087. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Terada N, Nomura T, Takahashi R, Lee SD, Park JH, et al. Effect of formaldehyde on the expression of adhesion molecules in nasal microvascular endothelial cells: the role of formaldehyde in the pathogenesis of sick building syndrome. Clin Exp Allergy. 2002;32(2):287–295. doi: 10.1046/j.1365-2222.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- Kin K, Miyagawa S, Fukushima S, Shirakawa Y, Torikai K, Shimamura K, et al. 2012Tissue- and plasma-specific microRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc 15e000745; 10.1161/JAHA.112.000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong AP, Xiao K, Choi KC, Wang G, Chan MH, Ho CS, et al. Associations between microRNA (miR-21, 126, 155 and 221), albuminuria and heavy metals in Hong Kong Chinese adolescents. Clin Chim Acta. 2012;413(13–14):1053–1057. doi: 10.1016/j.cca.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Kong W, He L, Richards EJ, Challa S, Xu CX, Permuth-Wey J, et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene. 2014;33(6):679–689. doi: 10.1038/onc.2012.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Gill KD. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol. 2009;83(11):965–978. doi: 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- Lau P, de Strooper B. Dysregulated microRNAs in neurodegenerative disorders. Semin Cell Dev Biol. 2010;21(7):768–773. doi: 10.1016/j.semcdb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Leucci E, Zriwil A, Gregersen LH, Jensen KT, Obad S, Bellan C, et al. Inhibition of miR-9 de-represses HuR and DICER1 and impairs Hodgkin lymphoma tumour outgrowth in vivo. Oncogene. 2012;31(49):5081–5089. doi: 10.1038/onc.2012.15. [DOI] [PubMed] [Google Scholar]
- Levänen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131(3):894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GY, Lee HY, Shin HS, Kim HY, Lim CH, Lee BH.2007Identification of gene markers for formaldehyde exposure in humans. Environ Health Perspect 1151460–1466.; 10.1289/ehp.10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu Y, Song G, Liu N. A microarray for microRNA profiling in spermatozoa from adult men living in an environmentally polluted site. Bull Environ Contam Toxicol. 2012a;89(6):1111–1114. doi: 10.1007/s00128-012-0827-0. [DOI] [PubMed] [Google Scholar]
- Li X, Shi Y, Wei Y, Ma X, Li Y, Li R. Altered expression profiles of microRNAs upon arsenic exposure of human umbilical vein endothelial cells. Environ Toxicol Pharmacol. 2012b;34(2):381–387. doi: 10.1016/j.etap.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Liang P, Lv C, Jiang B, Long X, Zhang P, Zhang M, et al. MicroRNA profiling in denatured dermis of deep burn patients. Burns. 2012;38(4):534–540. doi: 10.1016/j.burns.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Liu C, Duan W, Li R, Xu S, Zhang L, Chen C, et al. 2013Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity. Cell Death Dis 4e676; 10.1038/cddis.2013.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, et al. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett. 2011;585(9):1363–1367. doi: 10.1016/j.febslet.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Alexandrov PN. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol Neurobiol. 2012;46(1):11–19. doi: 10.1007/s12035-012-8234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Dua P, Pogue AI, Eicken C, Hill JM. Upregulation of micro RNA-146a (miRNA-146a), a marker for inflammatory neurodegeneration, in sporadic Creutzfeldt-Jakob disease (sCJD) and Gerstmann-Straussler-Scheinker (GSS) syndrome. J Toxicol Environ Health A. 2011;74(22–24):1460–1468. doi: 10.1080/15287394.2011.618973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101(9):1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv SQ, Kim YH, Giulio F, Shalaby T, Nobusawa S, Yang H, et al. Genetic alterations in microRNAs in medulloblastomas. Brain Pathol. 2012;22(2):230–239. doi: 10.1111/j.1750-3639.2011.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala, Pan E, Mestdagh P, Muth D, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5(7):583–589. doi: 10.4161/epi.5.7.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Padbury JF, Marsit CJ.2011miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS One 66e21210; 10.1371/journal.pone.0021210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci G, Radmacher MD, Maharry K, Mrózek K, Ruppert AS, Paschka P, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7(5):432–439. doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- Miller MR, Shaw CA, Langrish JP. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8(4):577–602. doi: 10.2217/fca.12.43. [DOI] [PubMed] [Google Scholar]
- Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283(44):29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology. 1995;51(6):435–445. doi: 10.1002/tera.1420510609. [DOI] [PubMed] [Google Scholar]
- Motta V, Angelici L, Nordio F, Bollati V, Fossati S, Frascati F, et al. Integrative analysis of miRNA and inflammatory gene expression after acute particulate matter exposure. Toxicol Sci. 2013;132(2):307–316. doi: 10.1093/toxsci/kft013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16(3):223–229. doi: 10.1016/j.ceb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Nagano T, Higashisaka K, Kunieda A, Iwahara Y, Tanaka K, Nagano K, et al. 2013Liver-specific microRNAs as biomarkers of nanomaterial-induced liver damage. Nanotechnology 2440405102; 10.1088/0957-4484/24/40/405102 [DOI] [PubMed] [Google Scholar]
- Nagel R, le Sage C, Diosdado B, van der Waal M, Oude Vrielink JA, Bolijn A, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68(14):5795–5802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Taccioli C, Palatini J, Fernandez-Cymering C, Cui R, Kim T, et al. Loss of miR-125b-1 contributes to head and neck cancer development by dysregulating TACSTD2 and MAPK pathway. Oncogene. 2014;33(6):702–712. doi: 10.1038/onc.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nana-Sinkam SP, Hunter MG, Nuovo GJ, Schmittgen TD, Gelinas R, Galas D, et al. Integrating the microRNome into the study of lung disease. Am J Respir Crit Care Med. 2009;179(1):4–10. doi: 10.1164/rccm.200807-1042PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, et al. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23(2):265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota D, Mimori K, Yokobori T, Iwatsuki M, Kataoka A, Masuda N, et al. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int J Oncol. 2011;38(4):955–962. doi: 10.3892/ijo.2011.926. [DOI] [PubMed] [Google Scholar]
- Overton JH, Kimbell JS, Miller FJ. Dosimetry modeling of inhaled formaldehyde: the human respiratory tract. Toxicol Sci. 2001;64(1):122–134. doi: 10.1093/toxsci/64.1.122. [DOI] [PubMed] [Google Scholar]
- Pallante P, Visone R, Ferracin M, Ferraro A, Berlingieri MT, Troncone G, et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer. 2006;13(2):497–508. doi: 10.1677/erc.1.01209. [DOI] [PubMed] [Google Scholar]
- Palma CA, Al Sheikha D, Lim TK, Bryant A, Vu TT, Jayaswal V, et al. 2014MicroRNA-155 as an inducer of apoptosis and cell differentiation in Acute Myeloid Leukaemia. Mol Cancer 13179; 10.1186/1476-4598-13-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, et al. 2007microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol 82R27; 10.1186/gb-2007-8-2-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TP, Percy ME, et al. Characterization of an NF-κB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J Inorg Biochem. 2009;103(11):1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Polikepahad S, Knight JM, Naghavi AO, Oplt T, Creighton CJ, Shaw C, et al. Proinflammatory role for let-7 microRNAS in experimental asthma. J Biol Chem. 2010;285(39):30139–30149. doi: 10.1074/jbc.M110.145698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, et al. 2011Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure–response relationships. Environ Health Perspect 1191616–1621.; 10.1289/ehp.1103639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta M, Zumeta E. Implementing the Stockholm Treaty on Persistent Organic Pollutants [Editorial]. Occup Environ Med. 2002;59(10):651–652. doi: 10.1136/oem.59.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopec SD, Watson JD, Waggott DM, Smith AB, Wu AH, Okey AB, et al. Systematic evaluation of medium-throughput mRNA abundance platforms. RNA. 2013;19(1):51–62. doi: 10.1261/rna.034710.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian ZR, Asa SL, Siomi H, Siomi MC, Yoshimoto K, Yamada S, et al. Overexpression of HMGA2 relates to reduction of the let-7 and its relationship to clinicopathological features in pituitary adenomas. Mod Pathol. 2009;22(3):431–441. doi: 10.1038/modpathol.2008.202. [DOI] [PubMed] [Google Scholar]
- Qu P, Roberts J, Li Y, Albrecht M, Cummings OW, Eble JN, et al. Stat3 downstream genes serve as biomarkers in human lung carcinomas and chronic obstructive pulmonary disease. Lung Cancer. 2009;63:341–347. doi: 10.1016/j.lungcan.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager JE, Moeller BC, Doyle-Eisele M, Kracko D, Swenberg JA, Fry RC.2013Formaldehyde and epigenetic alterations: microRNA changes in the nasal epithelium of nonhuman primates. Environ Health Perspect 121339–344.; 10.1289/ehp.1205582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager JE, Moeller BC, Miller SK, Kracko D, Doyle-Eisele M, Swenberg JA, et al. Formaldehyde-associated changes in microRNAs: tissue and temporal specificity in the rat nose, white blood cells, and bone marrow. Toxicol Sci. 2014;138(1):36–46. doi: 10.1093/toxsci/kft267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager JE, Smeester L, Jaspers I, Sexton KG, Fry RC.2011Epigenetic changes induced by air toxics: formaldehyde exposure alters miRNA expression profiles in human lung cells. Environ Health Perspect 119494–500.; 10.1289/ehp.1002614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Qian ZR, Wang EL, Sultana R, Kudo E, Nakasono M, et al. Frequent overexpression of HMGA1 and 2 in gastroenteropancreatic neuroendocrine tumours and its relationship to let-7 downregulation. Br J Cancer. 2009;100(3):501–510. doi: 10.1038/sj.bjc.6604883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitoharju E, Lyytikäinen LP, Levula M, Oksala N, Mennander A, Tarkka M, et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219(1):211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, Løvendorf MB, Ahler CB, Svensson L, et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118(22):5891–5900. doi: 10.1182/blood-2011-06-358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127(1–2):27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Saetrom P, Heale BS, Snøve O, Jr, Aagaard L, Alluin J, Rossi JJ. Distance constraints between microRNA target sites dictate efficacy and cooperativity. Nucleic Acids Res. 2007;35:2333–2342. doi: 10.1093/nar/gkm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M, Miki Y, Masuda M, Hata S, Shibahara Y, Hirakawa H, et al. LIN28: a regulator of tumor-suppressing activity of let-7 microRNA in human breast cancer. J Steroid Biochem Mol Biol. 2012;131(3–5):101–106. doi: 10.1016/j.jsbmb.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Scapoli L, Palmieri A, Lo Muzio L, Pezzetti F, Rubini C, Girardi A, et al. MicroRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int J Immunopathol Pharmacol. 2010;23(4):1229–1234. doi: 10.1177/039463201002300427. [DOI] [PubMed] [Google Scholar]
- Schembri F, Sridhar S, Perdomo C, Gustafson AM, Zhang X, Ergun A, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci USA. 2009;106(7):2319–2324. doi: 10.1073/pnas.0806383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte JH, Marschall T, Martin M, Rosenstiel P, Mestdagh P, Schlierf S, et al. Deep sequencing reveals differential expression of microRNAs in favorable versus unfavorable neuroblastoma. Nucleic Acids Res. 2010;38(17):5919–5928. doi: 10.1093/nar/gkq342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan ZX, Lin QX, Fu YH, Deng CY, Zhou ZL, Zhu JN, et al. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem Biophys Res Commun. 2009;381(4):597–601. doi: 10.1016/j.bbrc.2009.02.097. [DOI] [PubMed] [Google Scholar]
- Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, et al. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Singh NP, Singh UP, Guan H, Nagarkatti P, Nagarkatti M.2012Prenatal exposure to TCDD triggers significant modulation of microRNA expression profile in the thymus that affects consequent gene expression. PLoS One 79e45054; 10.1371/journal.pone.0045054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MK, Park YK, Ryu JC. Polycyclic aromatic hydrocarbon (PAH)-mediated upregulation of hepatic microRNA-181 family promotes cancer cell migration by targeting MAPK phosphatase-5, regulating the activation of p38 MAPK. Toxicol Appl Pharmacol. 2013;273(1):130–139. doi: 10.1016/j.taap.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Soto AM, Brisken C, Schaeberle C, Sonnenschein C. Does cancer start in the womb? Altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors. J Mammary Gland Biol Neoplasia. 2013;18(2):199–208. doi: 10.1007/s10911-013-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA. 2004;101(27):10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stánitz E, Juhász K, Tóth C, Gombos K, Natali PG, Ember I. Evaluation of MicroRNA expression pattern of gastric adenocarcinoma associated with socioeconomic, environmental and lifestyle factors in northwestern Hungary. Anticancer Res. 2013;33(8):3195–3200. [PubMed] [Google Scholar]
- Steele G, Stehr-Green P, Welty E. Estimates of the biologic half-life of polychlorinated biphenyls in human serum [Letter]. N Engl J Med. 1986;314(14):926–927. doi: 10.1056/NEJM198604033141418. [DOI] [PubMed] [Google Scholar]
- Sturchio E, Colombo T, Boccia P, Carucci N, Meconi C, Minoia C, et al. Arsenic exposure triggers a shift in microRNA expression. Sci Total Environ. 2014;472:672–680. doi: 10.1016/j.scitotenv.2013.11.092. [DOI] [PubMed] [Google Scholar]
- Sweeney LM, Okolica MR, Gut CP, Jr, Gargas ML. Cancer mode of action, weight of evidence, and proposed cancer reference value for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Regul Toxicol Pharmacol. 2012;64(2):205–224. doi: 10.1016/j.yrtph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yokota S, Tatsumi N, Fukami T, Yokoi T, Nakajima M. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharmacol. 2013;272(1):154–160. doi: 10.1016/j.taap.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Oishi S. Disposition of orally administered 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect. 2000;108:931–935. doi: 10.1289/ehp.00108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64(11):3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116(3):258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- Tilghman SL, Bratton MR, Segar HC, Martin EC, Rhodes LV, Li M, et al. 2012Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS One 73e32754; 10.1371/journal.pone.0032754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CH. Cardiovascular disease in arsenic-exposed subjects living in the arseniasis-hyperendemic areas in Taiwan. Atherosclerosis. 2008;199(1):12–18. doi: 10.1016/j.atherosclerosis.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Tsukimori K, Tokunaga S, Shibata S, Uchi H, Nakayama D, Ishimaru T, et al. 2008Long-term effects of polychlorinated biphenyls and dioxins on pregnancy outcomes in women affected by the Yusho incident. Environ Health Perspect 116626–630.; 10.1289/ehp.10686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pottelberge GR, Mestdagh P, Bracke KR, Thas O, van Durme YM, Joos GF, et al. MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(7):898–906. doi: 10.1164/rccm.201002-0304OC. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316(5824):575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Voellenkle C, van Rooij J, Cappuzzello C, Greco S, Arcelli D, Di Vito L, et al. MicroRNA signatures in peripheral blood mononuclear cells of chronic heart failure patients. Physiol Genomics. 2010;42(3):420–426. doi: 10.1152/physiolgenomics.00211.2009. [DOI] [PubMed] [Google Scholar]
- Vogt M, Munding J, Grüner M, Liffers ST, Verdoodt B, Hauk J, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458(3):313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waliszewski SM, Aguirre AA, Infanzon RM, Silva CS, Siliceo J. Organochlorine pesticide levels in maternal adipose tissue, maternal blood serum, umbilical blood serum, and milk from inhabitants of Veracruz, Mexico. Arch Environ Contam Toxicol. 2001;40(3):432–438. doi: 10.1007/s002440010194. [DOI] [PubMed] [Google Scholar]
- Wang C, Yang C, Chen X, Yao B, Yang C, Zhu C, et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin Chem. 2011;57(12):1722–1731. doi: 10.1373/clinchem.2011.169714. [DOI] [PubMed] [Google Scholar]
- Wang F, Liu W, Jin Y, Wang F, Ma J.2014Prenatal and neonatal exposure to perfluorooctane sulfonic acid results in aberrant changes in miRNA expression profile and levels in developing rat livers. Environ Toxicol; 10.1002/tox.21949[Online 13 January 2014] [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L.2012Serum microRNA signatures identified by Solexa sequencing predict sepsis patients’ mortality: a prospective observational study. PLoS One 76e38885; 10.1371/journal.pone.0038885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Zhang W, Jin Y, Dai J. Association of perfluorooctanoic acid with HDL cholesterol and circulating miR-26b and miR-199-3p in workers of a fluorochemical plant and nearby residents. Environ Sci Technol. 2012;46(17):9274–9281. doi: 10.1021/es300906q. [DOI] [PubMed] [Google Scholar]
- Wang X, Ha T, Liu L, Zou J, Zhang X, Kalbfleisch J, et al. Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2013;97(3):432–442. doi: 10.1093/cvr/cvs356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Z, He C, Wang D, Yuan X, Chen J, et al. MicroRNAs expression signatures are associated with lineage and survival in acute leukemias. Blood Cells Mol Dis. 2010;44(3):191–197. doi: 10.1016/j.bcmd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt K, Bergink V, Burgerhout KM, Pescatori M, Wijkhuijs A, Drexhage HA. Down-regulation of inflammation-protective microRNAs 146a and 212 in monocytes of patients with postpartum psychosis. Brain Behav Immun. 2013;29:147–155. doi: 10.1016/j.bbi.2012.12.018. [DOI] [PubMed] [Google Scholar]
- White NM, Bao TT, Grigull J, Youssef YM, Girgis A, Diamandis M, et al. miRNA profiling for clear cell renal cell carcinoma: biomarker discovery and identification of potential controls and consequences of miRNA dysregulation. J Urol. 2011;186(3):1077–1083. doi: 10.1016/j.juro.2011.04.110. [DOI] [PubMed] [Google Scholar]
- Wild C. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, et al. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326(5959):1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Liang R, So CC, Jin DY, Costello JF, Chim CS. Epigenetic silencing of MIR203 in multiple myeloma. Br J Haematol. 2011;154(5):569–578. doi: 10.1111/j.1365-2141.2011.08782.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang T, Li X, Yang Q, Liu R, Huang J, et al. Alteration of serum miR-206 and miR-133b is associated with lung carcinogenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Toxicol Appl Pharmacol. 2013;267(3):238–246. doi: 10.1016/j.taap.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J, Zhao JJ, et al. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117(13):2842–2852. doi: 10.1002/cncr.25860. [DOI] [PubMed] [Google Scholar]
- Xi S, Yang M, Tao Y, Xu H, Shan J, Inchauste S, et al. 2010Cigarette smoke induces C/EBP-β-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS One 510e13764; 10.1371/journal.pone.0013764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131(1):146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Xu CC, Han WQ, Xiao B, Li NN, Zhu DL, Gao PJ. Differential expression of microRNAs in the aorta of spontaneously hypertensive rats [in Chinese]. Sheng Li Xue Bao. 2008;60(4):553–560. [PubMed] [Google Scholar]
- Yamagishi M, Nakano K, Miyake A, Yamochi T, Kagami Y, Tsutsumi A, et al. Polycomb-mediated loss of miR-31 activates NIK-dependent NF-κB pathway in adult T cell leukemia and other cancers. Cancer Cell. 2012;21(1):121–135. doi: 10.1016/j.ccr.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Singh A, Sava F, Pui M, Tebbutt SJ, Carlsten C.2013MicroRNA expression in response to controlled exposure to diesel exhaust: attenuation by the antioxidant N-acetylcysteine in a randomized crossover study. Environ Health Perspect 121670–675.; 10.1289/ehp.1205963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer cell. 2006;9(3):189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Yi C, Wang Q, Wang L, Huang Y, Li L, Liu L, et al. MiR-663, a microRNA targeting p21(WAF1/CIP1), promotes the proliferation and tumorigenesis of nasopharyngeal carcinoma. Oncogene. 2012;31(41):4421–4433. doi: 10.1038/onc.2011.629. [DOI] [PubMed] [Google Scholar]
- Yuchuan H, Ya D, Jie Z, Jingqiu C, Yanrong L, Dongliang L, et al. Circulating miRNAs might be promising biomarkers to reflect the dynamic pathological changes in smoking-related interstitial fibrosis. Toxicol Ind Health. 2014;30(2):182–191. doi: 10.1177/0748233712452606. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- Zhang B, Pan X.2009RDX induces aberrant expression of microRNAs in mouse brain and liver. Environ Health Perspect 117231–240.; 10.1289/ehp.11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14(13):1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pan T, Zhong X, Cheng C. Nicotine upregulates microRNA-21 and promotes TGF-β-dependent epithelial-mesenchymal transition of esophageal cancer cells. Tumour Biol. 2014;35(7):7063–7072. doi: 10.1007/s13277-014-1968-z. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan LX, Wu QN, Du ZM, Chen J, Liao DZ, et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71(10):3552–3562. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]


