Abstract
Bacterial infection is a major cause of morbidity affecting outcome following burn and inhalation injury. While experimental burn and inhalation injury animal models have suggested that mediators of cell damage and inflammation increase the risk of infection, few studies have been done on humans. This is a prospective, observational study of patients admitted to the North Carolina Jaycee Burn Center at the University of North Carolina who were intubated and on mechanical ventilation for treatment of burn and inhalational injury. Subjects were enrolled over a 2-yr period and followed till discharge or death. Serial bronchial washings from clinically indicated bronchoscopies were collected and analyzed for markers of tissue injury and inflammation. These include damage-associated molecular patterns (DAMPs) such as hyaluronic acid (HA), double-stranded DNA (dsDNA), heat-shock protein 70 (HSP-70), and high-mobility group protein B-1 (HMGB-1). The study population was comprised of 72 patients who had bacterial cultures obtained for clinical indications. Elevated HA, dsDNA, and IL-10 levels in bronchial washings obtained early (the first 72 h after injury) were significantly associated with positive bacterial respiratory cultures obtained during the first 14 days postinjury. Independent of initial inhalation injury severity and extent of surface burn, elevated levels of HA dsDNA and IL-10 in the central airways obtained early after injury are associated with subsequent positive bacterial respiratory cultures in patients intubated after acute burn/inhalation injury.
Keywords: inhalational injury, burn injury, innate immunology, respiratory infection, acute lung injury
bacterial infection is a major risk factor for mortality following burn and inhalation injury. Experimental models suggest that direct cell damage, edema, inflammation, and immunologic changes occur in the respiratory tract after these injuries (17, 19), and bacterial pneumonia has been linked to mortality in patients with burns and inhalation injury (9). We have previously shown that surface burns affect expression of Toll-like receptors (TLRs) on immune cells and impact systemic immunity with a resulting bias towards immunosuppressive IL-10 production (5, 21). However, few studies have explored the relationship between markers of tissue damage and immune response in human airways to the impact on host defense.
Damage-associated molecular patterns (DAMPs) are molecules with intracellular functions that are released by cells and tissues upon various types of injury, typically binding with low affinity to pattern-recognition receptors on cell surfaces and exerting complex effects on inflammation and immunity (16). Specific DAMPs known to interact with TLRs include the extracellular matrix protein hyaluronic acid (HA), which can also interact with CD44 to trigger activation of the inflammasome cascade; the molecular chaperone heat shock proteins (HSP); nucleic acids, which may interact with TLR9 to activate neutrophils (11, 29); and the nuclear chromatin-associated protein, high-mobility box group-1 (HMGB-1) (16), which is responsible for the inflammatory response to cell necrosis (13). It has been postulated that DAMPs can polarize macrophages and other innate cells towards an IL-10 immunosuppressive phenotype (20).
The clinical practice of serial bronchoscopy for supportive care after inhalational injury (6) presents a unique opportunity to obtain lower airway secretions from patients for investigation of the relationships among mediators and clinical outcomes of interest. As described previously, we established a repository of bronchoscopy samples for analysis of airway mediators and their relationships with clinical outcomes and reported that high IL-10 levels in early airway secretions are associated with acute lung injury (ALI) in a cohort of 43 consecutive burn/inhalation injury patients (14). We hypothesized that there would be an association between pulmonary DAMPs and IL-10 with pulmonary bacterial infection. Therefore, in the current study we used an expanded sample repository and clinical database to explore associations among specific DAMPs, inflammation, and bacterial infection. We report data showing that elevated levels of HA, dsDNA, and IL-10 observed early in the airway are associated with subsequent bacterial infection.
MATERIALS AND METHODS
Study design.
This is a single-center, prospective observational study of intubated patients with inhalation injury and burns admitted to the North Carolina Jaycee Burn Center at the University of North Carolina at Chapel Hill (UNC) over a 2-yr period. Intubated patients underwent mechanical ventilation and one or more bronchoscopies as described previously (14). Per clinical protocol, all patients in the study underwent at least one bronchoscopy at admission. Since indications for subsequent bronchoscopies were determined by individual clinical factors, the total number and timing of bronchoscopies varied among patients studied.
For the research protocol, patients were included if they were intubated and mechanically ventilated for known or suspected inhalational injury and underwent at least one clinically indicated bronchoscopy in the first 72 h after admission. Patients were excluded for 1) history of chronic lung disease including asthma before injury; 2) history of immune deficiency; or 3) recent (past 2 wk) use of inhaled or systemic corticosteroids. Clinical data including demographics and percent total body surface area burn (TBSA) were collected at admission. Additional clinical data including results of respiratory cultures were obtained as part of routine clinical care at the time of each bronchoscopy. After use of bronchial washings for clinical care purposes, leftover material was processed for the research protocol and stored for mediator assays described below.
Levels of “early” (<72 h postinjury) mediators measured in bronchial washings were compared with bacterial culture data during the first 14 days postinjury. Study sample size was determined by the number of consecutive eligible patients admitted and consented to participate during the 2-yr period. The first 43 patients in this series of consecutive patients are the same ones in our previous study (14), and subsequent patients are new to the current report.
Since bronchoscopic samples used for the study would otherwise be discarded, samples were collected and processed but not stored in the repository until written informed consent was obtained from the patient's legal authorized representative (LAR). Subjects who improved subsequently were given the opportunity to confirm or deny the LAR's informed consent. The study was approved by the UNC Biomedical Institutional Review Board.
Processing of bronchial washings and mediator assays.
Bronchial washing specimens were transported on ice and processed within 5 h of specimen collection. Briefly, samples were centrifuged at 300 g and aliquots of the resultant cell-free supernatant were stored at −80°C for subsequent mediator measurements. dsDNA was quantified using QuantIt PicoGreen dsDNA Assay Kit (Invitrogen, Eugene, OR), a fluorescent nucleic acid stain. Commercially available ELISA were used to quantify HA (R&D Systems, Minneapolis, MN), HSP-70 (Enzo Life Sciences, Farmingdale, NY), and HMBG-1 (IBL International; Hamburg, Germany). In addition, the same panel of cytokines studied previously (IL-1β, IL-6, IL-8, IL-10, IL-12 p70, IFNγ, and TNF-α) were measured using a multiplex ELISA platform (MesoScale Discovery, Gaithersburg, MD), and transforming growth factor-β1 (TGF-β1) was measured using a commercial ELISA kit (R&D Systems) (14). All assays were run according to the manufacturer's instructions.
Statistics.
Subjects underwent one to three bronchoscopies during the initial 72 h postinjury. To reduce the data to a single point per mediator and patient, if multiple samples were generated for a patient during the first 72 h, the mediator data were averaged. We used multivariate regression analyses to test for an association between DAMPS or cytokines in “early” (<72 h) bronchial washings and clinical outcomes of infection at any point during the 14 days postinjury. Confounders included in the model were bronchoscopic assessment of initial injury severity [see scoring system we have previously published in Jones et al. (14)], age, body mass index (BMI), and percent body surface area burn included in the model. Specifically, we fitted the following logistic regression model:
where Y = 1 indicates positive bacterial respiratory cultures and X1 indicates the primary exposure variable of interests. P < 0.05 was considered significant throughout. Statistical significance (P) and degree of fit (r2) were quantified using t-test, ANOVA, or linear regression line fitting where appropriate using GraphPad Prism version 4.2 for Windows (GraphPad Software, San Diego CA: www.graphpad.com).
RESULTS
Patient characteristics.
There were 102 patients included in the sample collection and repository. However, patients who had no respiratory bacterial cultures sent during the first 14 days postinjury as part of clinical care were excluded from analysis (n = 30). For dsDNA and the cytokines, the sample size was thus 72. For HSP-70, HA, and HMGB-1, the sample size was 69 because these covariates had more missing values. Demographic and outcomes data for the patients analyzed are shown in Table 1, with the distribution of collection and positive bacterial cultures in Fig. 1, B and C. Age and gender were similar between the subgroups who had positive bacterial cultures vs. negative bacterial cultures. Roughly half of the 72 patients had at least one positive bacterial culture from bronchial washings at some point during the 14 days postinjury, and this subgroup is referred to as “infected.” Pseudomonas, Acinetobacter, Escherichia coli, Klebsiella, Enterobacter, Hemophilus influenza, Streptococcus pneumonia, and Staphylococcus aureus were all represented in the patient cohort.
Table 1.
Demographic characteristics and clinical outcomes of patients in the study, for uninfected vs. infected patients
| Age | Gender (%male) | %TBSA Burn | Days on Ventilator | Days in Hospital | Mortality | |
|---|---|---|---|---|---|---|
| Uninfected | ||||||
| Mean | 41 | 70 | 7 | 8 | 21 | 9 |
| IQR | (27, 58) | (1, 22) | (1, 30) | (8, 50) | ||
| Infected | ||||||
| Mean | 46 | 60 | 16 | 43 | 50 | 17 |
| IQR | (30, 56) | (2, 29) | (19, 63) | (27, 79) | ||
| P | 0.71 | 0.15 | 0.13 | <0.001 | 0.02 | 0.01 |
Data are shown as median [interquartile range (IQR)]. TBSA, total body surface area. Statistical significance (P) was quantified using one-way ANOVA.
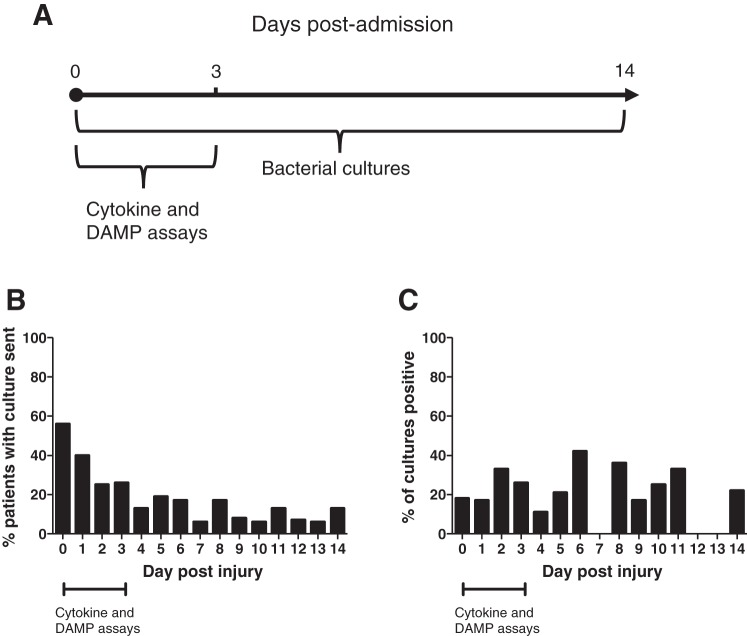
Fig. 1.
Serial bronchial samples were collected for analysis over time, with a wide distribution of bacterial infection frequency postinjury. Serial bronchial washings from clinically indicated bronchoscopies were collected. With the use of a multivariate regression model, damage-associated molecular patterns (DAMPs) and cytokines in early washings were tested for association with bacterial respiratory infection during the first 14 days after injury (A), with the percentage of patients in study who had a bronchial washing sent for culture by day postinjury during the first 14 days after injury (B), and the percentage of bronchial washing cultures that were positive for any bacterial pathogen by day postinjury (C).
For the study population as a whole, length of hospital stay after injury ranged from 2 to 352 days (mean 47 days). The number of days on the ventilator was variable and averaged 32 days. Time on the ventilator was associated not only by the severity of inhalation injury or pulmonary status but also by percent total body surface area (TBSA) and comorbid conditions. Mortality was for the study population was 12%. Mean percent TBSA burns did not differ significantly between infected and uninfected subgroups, but number of days in hospital, number of days on ventilator, and mortality were all significantly higher in the infected subgroup (Table 1).
Relationship of DAMPs in early bronchial washings to subsequent bacterial infection.
In the multivariate regression model, each of the measured DAMPs in early mainstem bronchial washings was tested for association with bacterial respiratory infection during the first 14 days after injury. Percent TBSA burn, initial airway injury score, BMI, and age were treated as potential confounders in the model. Elevated HA and dsDNA were significantly associated with bacterial infection in the model (P < 0.05), whereas HSP-70 and HMGB-1 were not. For descriptive purposes, data for HA and dsDNA in infected vs. uninfected groups are shown in Fig. 2 and Table 2. We also observed that early (patients with positive bacterial cultures within the first 72 h) pulmonary bacterial infection correlates with an increased HA amounts but not an increased dsDNA amount (Fig. 2). Early levels of HA and dsDNA in bronchial washings from all patients were statistically significantly correlated with each other (Fig. 3).
Fig. 2.

Elevated hyaluronic acid (HA) and double-stranded DNA (dsDNA) were significantly associated with subsequent bacterial infection. Serial bronchial washings from clinically indicated bronchoscopies were collected. Elevated HA (A) and dsDNA (B) were significantly associated with bacterial infection in the model, in all infected patients and in patients with positive bacterial culture within the first 72 h (“infected <72 h”), whereas heat shock protein-70 (HSP-70) and high-mobility group protein B-1 (HMGB-1) were not (not shown). Statistical significance (P) was quantified using t-test.
Table 2.
Levels of DAMPs in early bronchial washings in infected vs. uninfected patients
| HA, ng/ml | dsDNA, ng/ml | HSP-70, ng/ml | HMGB-1, ng/ml | |
|---|---|---|---|---|
| Uninfected | ||||
| Mean | 217 | 184 | 5.5 | 27 |
| IQR | (57, 389) | (0, 3,054) | (1.6, 11.1) | (19, 74) |
| Infected | ||||
| Mean | 390 | 2,382 | 11.3 | 34 |
| IQR | (150, 467) | (820, 7,396) | (2.9, 15.1) | (23, 88) |
| P | 0.04 | 0.02 | 0.93 | 0.33 |
Data are shown as median (IQR). DAMPs, damage-associated molecular patterns; HA, hyaluronic acid; ds-DNA, double-stranded DNA; HSP-70, heat-shock protein-70; HMGB-1, high-mobility group protein B-1. P values are for association with infection in multivariate regression model.
Fig. 3.

Early levels of HA and dsDNA in bronchial washings were significantly correlated with each other. Serial bronchial washings from clinically indicated bronchoscopies were collected and analyzed for markers of tissue injury and inflammation. Each DAMP in early washings (average concentration over the first 72 h) was tested for association with each other during the first 14 days after injury. Statistical significance (P) and degree of fit (r2) was quantified using linear regression line fitting.
Relationship of cytokines in early bronchial washings to subsequent bacterial infection.
Each of the cytokines measured was also tested for association with bacterial respiratory infection in the multivariate regression model. Of this group of cytokines, only IL-10 levels associated significantly with infection (Table 3). In our previous report, the ratio of IL-10/IL-12p70 in early bronchial washings was elevated in patients with ALI (14), thus we assessed this parameter here. In addition to IL-10 being significantly associated with infection in the multivariate regression model, the ratio of IL-10/IL-12p70 was also significantly higher for infected patients (Fig. 4). Levels of IL-10 did not significantly correlate with levels of any of the DAMPs measured (data not shown).
Table 3.
Levels of cytokines in early bronchial washings in infected vs. uninfected patients
| IL-1β, pg/ml | IL-6, pg/ml | IL-8, μg/ml | IL-10, pg/ml | IL-12p70 | IFNγ, pg/ml | TNF-α | |
|---|---|---|---|---|---|---|---|
| Uninfected | |||||||
| Mean | 260 | 777 | 7,079 | 45 | 21 | 39 | 188 |
| IQR | (63, 1,929) | (1,336, 25,268) | (1,336, 25,268) | (28, 75) | (16, 29) | (31, 62) | (50, 491) |
| Infected | |||||||
| Mean | 568 | 691 | 11,796 | 74 | 15 | 34 | 232 |
| IQR | (117, 2,806) | (101, 1,663) | (3,425, 37,126) | (33, 94) | (11, 22) | (23, 88) | (94, 967) |
| P | 0.16 | 0.89 | 0.69 | 0.03 | 0.21 | 0.06 | 0.66 |
Data are shown as median (IQR). P values are for association with infection in the multivariate regression model.
Fig. 4.

The ratio of IL-10/IL-12p70 was significantly higher for infected patients compared with uninfected patients. Early IL-10 and IL-12 levels were measured within serial bronchial mainstem washes from patients with inhalational and burn injury and tested for association with later bacterial respiratory infection in all infected patients and in patients with positive bacterial culture within the first 72 h (“infected <72 h”). Statistical significance (P) was quantified using t-test.
DISCUSSION
Both infection and ALI are associated with morbidity and mortality following burns and inhalation injury (9, 17, 19). We previously reported that elevated IL-10 and reduced IL-12p70 in bronchial washings were statistically significantly associated with ALI following inhalation injury (14). It has been postulated that DAMPs released from damaged tissues drive immune dysfunction after injury (3, 16, 27). We therefore hypothesized that increased DAMPs released from damaged tissue would correlate with bacterial infection and IL-10/IL-12p70 levels. In bronchial washings obtained during the first 72 h after injury, elevated levels of HA, dsDNA, and the cytokine IL-10 were significantly associated with positive bacterial respiratory cultures obtained during the first 14 days postinjury. However, there was no correlation between early HA and dsDNA levels and the early IL-10/IL-12p70 levels.
A major determinant of immune cell recruitment and activity is the TLR family members expressed predominantly on innate immune cells (1, 2). The primary function of TLR is to be “pattern recognition receptors” for the innate immune system, capable of sensing molecules normally associated with microorganisms (1, 2). Endogenous ligands also exist for mammalian innate receptors with both necrotic and apoptotic cells able to induce cell signaling through TLR and other innate sensing molecules via release of endogenous ligands, known as DAMPS such as HA, dsDNA, HSP-70, and HMGB-1 (3, 27). We and others have shown that TLR expression and signaling is often altered after injury leading to hypo- or hyper-responsiveness (10, 18). Here we show that early levels of HA and dsDNA in bronchial washings were significantly correlated with each other and elevated levels of both are significantly associated with bacterial infection in patients, whereas HSP-70 and HMGB-1 are not.
Macrophages and neutrophils can be polarized into an anti-inflammatory phenotype due to TLR-signaling by DAMPS released from damaged tissue (4, 12, 15, 23, 31). These anti-inflammatory macrophages (M2) and neutrophils (N2) secrete high amounts of IL-10, a potent anti-inflammatory cytokine (5, 7, 22, 24), and have been implicated in the dysfunctional immune response to burn injury. Excessive IL-10 has been shown to be detrimental for bacterial clearance by attenuating protective proinflammatory cytokines, such as IL-12 (25, 26, 28), and in our prior report elevated IL-10 was associated with increased ALI risk after burn and inhalation injury (14).
We recently published that burned mice were highly susceptibility to Pseudomonas aeruginosa infection after wound inoculation, with an elevated IL-10/IL-12 ratio and a significant number of innate cells polarized to an IL-10+ IL-12− anti-inflammatory phenotype. This study also demonstrated that the susceptibility to P. aeruginosa wound infection could be reduced by administration of a TLR5 agonist, flagellin, immediately after burn injury, which was also associated with reversal of an observed IL-10+ IL-12− innate cell polarization. In addition, numerous studies have linked high circulating levels of IL-10 with poor outcomes following burn injury, sepsis, and other bacterial infections (8, 25, 26). Therefore, current literature and our earlier studies predicted that DAMP release from injury triggers a shift toward M2 or N2 polarized responses, resulting in an elevated IL-10/IL-12p70 ratio and a greater susceptibility to infection. However, this study suggests for the first time in humans that this is not a simple causal (early DAMP release → early IL-10 → bacterial infection) relationship. Our data also suggest that patients that develop early (<72 h) infections or already have bacteria in the airway trigger more DAMPs with the inhalation injury and more IL-10. While it appears that early levels of HA and dsDNA in bronchial washings were statistically significantly correlated with each other suggesting both are released after injury to similar extents, patients with early infections trend towards greater levels of HA compared with dsDNA. This finding suggests that the microbial flora may act in some way to exacerbate tissue damage and DAMP release. It is possible that these factors are unrelated responses to the same injury and further studies in experimental models may help sort among these possible interpretations. Ongoing studies will assess causative relationships among specific DAMPs, the composition of the DAMPs [for example, does the dsDNA contain mitochondrial DNA, which has been recognized as a potent DAMP in the induction of ALI (30)], immune polarization, and bacterial infections in the setting of burn and inhalational injuries.
Our study is observational and relies on our clinical practice to use serial flexible bronchoscopy for assessment and treatment of airway obstruction. Thus multiple uncontrolled factors other than DAMPs or cytokines most likely contribute to infection risk in our patient population. We attempted to reduce this possibility by including percent TBSA burn, bronchoscopically determined visual severity of initial inhalation injury, age, and BMI as confounders in the statistical model, since these variables are reasonably expected to influence infection risk. Another limitation of this study is our reliance on clinical indications for bronchoscopy and cultures of bronchoscopically derived specimens. To reduce this limitation for the current analysis, we excluded 12 patients who were part of the biorepository but in whom bacterial respiratory cultures were not obtained during the timeframe of interest (the first 14 days postinjury). As a result, our study population was likely biased toward those patients in whom there was some clinical suspicion for infection. A systematic collection of culture data would be needed to eliminate this bias, although this will require careful design to minimize patient risk in the critical care setting.
In summary, this study supports the further investigation of HA, dsDNA, and IL-10 as either predictors of later bacterial infection or having a role in the pathophysiology of infection in the setting of burn/inhalation injury. Elucidating the complex interplay among products of cell damage, cytokines, and bacterial infection susceptibility will help us design new diagnostic and therapeutic strategies for treatment of these patients. Since infectious complications are a main cause of mortality after burn and inhalation injury, it would be extremely helpful to identify biomarkers that might identify the risk for infection, infection itself, and drug targets to improve control of invading pathogens. While DAMPs have been postulated to play a role in various inflammatory conditions, this is the first study to correlate early, local organ-specific DAMP release with later occurrence of bacterial infection in human subjects.
GRANTS
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) through Grants 1UL1TR0011111 and KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.M., S.J., I.J., D.B.P., B.A.C., and T.L.N. conception and design of research; R.M., S.J., Y.P., H.Z., and T.L.N. analyzed data; R.M., I.J., D.B.P., and T.L.N. interpreted results of experiments; R.M. and T.L.N. prepared figures; R.M. and T.L.N. drafted manuscript; R.M., S.J., H.Z., I.J., D.B.P., B.A.C., and T.L.N. edited and revised manuscript; R.M., S.J., Y.P., H.Z., I.J., D.B.P., B.A.C., and T.L.N. approved final version of manuscript; S.J. performed experiments.
ACKNOWLEDGMENTS
We thank Paula C. Murphy for expert technical assistance. We also thank the North Carolina Jaycees Burn Center Respiratory Therapy team for help with specimen recovery and storage, and the North Carolina Children's Airway Center team (Kathy Abode, Clinical Coordinator) for assistance with pediatric procedures.
REFERENCES
- 1.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430: 257–263, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. Innate immune sensing of microbial infection: the mechanism and the therapeutic challenge. Wien Med Wochenschr 152: 547–551, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res 53: 11–24, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Cairns BA, Barnes CM, Mlot S, Meyer AA, Maile R. Toll-like receptor 2 and 4 ligation results in complex altered cytokine profiles early and late after burn injury. J Trauma 64: 1069–1077; discussion 1077–1068, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Carr JA, Phillips BD, Bowling WM. The utility of bronchoscopy after inhalation injury complicated by pneumonia in burn patients: results from the National Burn Repository. J Burn Care Res 30: 967–974, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MJ, Carroll C, He LK, Muthu K, Gamelli RL, Jones SB, Shankar R. Severity of burn injury and sepsis determines the cytokine responses of bone marrow progenitor-derived macrophages. J Trauma 62: 858–867, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Csontos C, Foldi V, Palinkas L, Bogar L, Roth E, Weber G, Lantos J. Time course of pro- and anti-inflammatory cytokine levels in patients with burns–prognostic value of interleukin-10. Burns 36: 483–494, 2010. [DOI] [PubMed] [Google Scholar]
- 9.D'Avignon LC, Hogan BK, Murray CK, Loo FL, Hospenthal DR, Cancio LC, Kim SH, Renz EM, Barillo D, Holcomb JB, Wade CE, Wolf SE. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns 36: 773–779, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Harter L, Mica L, Stocker R, Trentz O, Keel M. Increased expression of toll-like receptor-2 and -4 on leukocytes from patients with sepsis. Shock 22: 403–409, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma 24: 534–538, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med 15: 496–497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, Loike JD, Jenkins RE, Antoine DJ, Schwabe RF. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest 125: 539–550, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones SW, Zhou H, Ortiz-Pujols SM, Maile R, Herbst M, Joyner BL Jr, Zhang H, Kesic M, Jaspers I, Short KA, Meyer AA, Peden DB, Cairns BA, Noah TL. Bronchoscopy-derived correlates of lung injury following inhalational injuries: a prospective observational study. PLoS One 8: e64250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasten KR, Muenzer JT, Caldwell CC. Neutrophils are significant producers of IL-10 during sepsis. Biochem Biophys Res Commun 393: 28–31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuipers MT, van der Poll T, Schultz MJ, Wieland CW. Bench-to-bedside review: damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care 15: 235, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange M, Hamahata A, Traber DL, Esechie A, Jonkam C, Bansal K, Nakano Y, Traber LD, Enkhbaatar P. A murine model of sepsis following smoke inhalation injury. Biochem Biophys Res Commun 391: 1555–1560, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Martins PS, Brunialti MK, Martos LS, Machado FR, Assuncao MS, Blecher S, Salomao R. Expression of cell surface receptors and oxidative metabolism modulation in the clinical continuum of sepsis. Crit Care 12: R25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami K, Traber DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci 18: 125–129, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neely CJ, Maile R, Wang MJ, Vadlamudi S, Meyer AA, Cairns BA. Th17 (IFNgamma- IL17+) CD4+ T cells generated after burn injury may be a novel cellular mechanism for postburn immunosuppression. J Trauma 70: 681–690, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noel G, Wang Q, Schwemberger S, Hanson C, Giacalone N, Haar L, Ogle CK. Neutrophils, not monocyte/macrophages, are the major splenic source of postburn IL-10. Shock 36: 149–155, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol 189: 3439–3448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwacha MG, Chaudry IH, Alexander M. Regulation of macrophage IL-10 production postinjury via beta2 integrin signaling and the P38 MAP kinase pathway. Shock 20: 529–535, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol 162: 392–399, 1999. [PubMed] [Google Scholar]
- 26.Sun L, Guo RF, Newstead MW, Standiford TJ, Macariola DR, Shanley TP. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am J Respir Cell Mol Biol 41: 76–84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolle LB, Standiford TJ. Danger-associated molecular patterns (DAMPs) in acute lung injury. J Pathol 229: 145–156, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Hirakata Y, Izumikawa K, Miyazaki Y, Maesaki S, Tomono K, Yamada Y, Kohno S, Kamihira S. Prolonged survival of mice with Pseudomonas aeruginosa-induced sepsis by rIL-12 modulation of IL-10 and interferon-gamma. J Med Microbiol 49: 701–707, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34: 55–59, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol 214: 161–178, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]