Abstract
Rat pups prenatally exposed to nicotine (PNE) present apneic (lethal ventilatory arrest) responses during severe hypoxia. To clarify whether these responses are of central origin, we tested PNE effects on ventilation and diaphragm electromyography (EMGdi) during hypoxia in conscious rat pups. PNE produced apnea (lethal ventilatory arrest) identical to EMGdi silencing during hypoxia, indicating a central origin of this apneic response. We further asked whether PNE would sensitize bronchopulmonary C-fibers (PCFs), a key player in generating central apnea, with increase of the density and transient receptor potential cation channel subfamily V member 1 (TRPV1) expression of C-fibers/neurons in the nodose/jugular (N/J) ganglia and neurotrophic factors in the airways and lungs. We compared 1) ventilatory and pulmonary C-neural responses to right atrial bolus injection of capsaicin (CAP, 0.5 μg/kg), 2) bronchial substance P-immunoreactive (SP-IR) fiber density, 3) gene and protein expressions of TRPV1 in the ganglia, and 4) nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) protein in bronchoalveolar lavage fluid (BALF) and TrkA and TrkB genes in the ganglia between control and PNE pups. PNE markedly strengthened the PCF-mediated apneic response to CAP via increasing pulmonary C-neural sensitivity. PNE also enhanced bronchial SP-IR fiber density and N/J ganglia neural TRPV1 expression associated with increased gene expression of TrkA in the N/G ganglia and decreased NGF and BDNF in BALF. Our results suggest that PNE enhances PCF sensitivity likely through increasing PCF density and TRPV1 expression via upregulation of neural TrkA and downregulation of pulmonary BDNF, which may contribute to the PNE-promoted central apnea (lethal ventilatory arrest) during hypoxia.
Keywords: sudden infant death syndrome, maternal cigarette smoking, vagal afferents, central apnea, cardiorespiratory failure
we have recently found that prenatal nicotinic exposure (PNE) triggers the apneic response to hypoxia (5% O2 for 60 min), leading to a lethal ventilatory arrest in rat pups (68). However, the relevant mechanisms remain unknown. Animal studies suggest that PNE may increase airway resistance, including airway obstruction in proximal airways and reduction of airway conductance in monkeys (41, 42) and lambs (12, 40). Therefore, we tested whether the PNE-induced apnea and lethal ventilatory arrest during hypoxia in the rat pups resulted from central apnea or airway obstruction.
Because our preliminary results showed that the PNE-induced apnea was of central origin featured by lack of inspiratory drive from the central nervous system, we morphologically and functionally explored further the effects of PNE on bronchopulmonary C-fibers (PCFs). It is accepted that PCFs play a critical role in generating central apnea and lethal ventilatory arrest under pathophysiological conditions. PCFs traveling in the vagus nerve represent the majority of afferent nerves (75%) arising from the lungs and airways (7). Right atrial injection of capsaicin (CAP) to stimulate PCFs via action on transient receptor potential cation channel subfamily V member 1 (TRPV1), a nociceptive receptor, induces a brief central apnea (a few seconds) in different species (7, 58). Interestingly, this brief apnea becomes long lasting or even a lethal ventilatory arrest when PCFs are sensitized by hypoxia (58) or by pulmonary infection (36, 39). Pulmonary sensory fibers could be sensitized by nicotine (62), and the density of C-fibers (marked substance P, SP) innervating tracheal smooth muscles could be enhanced by prenatal exposure to cigarette smoke (57). Moreover, PNE has been reported to delay neural myelination in early life (24). These lines of information, along with the central origin of the apnea in our preliminary study, allow us to hypothesize that PNE could augment the PCF-mediated apneic response via an increase of the density of C-fiber expression and TRPV1 expression.
The nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are important members of the neurotrophin family and are responsible for the development, growth, maintenance, survival, and plasticity of certain target neurons (10). NGF and BDNF exist in the lungs during gestational and postnatal age (19). NGF could bind to p75NTR for low affinity and TrkA for high affinity, whereas BDNF predominantly binds to TrkB (21, 34). It has been reported that activation of the TrkA receptor of sensory neurons (21, 34) upregulates TRPV1 expression (2, 5, 63) and promotes synthesis of excitatory neuropeptides, such SP (29). On the other hand, 98% of nodose ganglion neurons (containing the cell bodies of PCFs) express TrkB (28), and BDNF is able to promote myelination of peripheral nerve (11, 31). Because of an overexpression of bronchial SP-immunoreactive (SP-IR) fibers by PNE in our pilot study, we hypothesized that PNE was able to alter NGF and BDNF expressions in lungs and TrkA/p75NTR and TrkB expressions in neurons of the nodose/jugular (N/J) ganglia.
MATERIALS AND METHODS
Male (13) and female (36) pathogen-free Sprague-Dawley rats (250–350 g) were purchased from Charles River Laboratories (Wilmington, MA) and quarantined for 2 wk before the experiments. The experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Pretreatment with PNE
The females were randomly designated to receive vehicle (n = 17) and nicotine (n = 19), respectively. Briefly (68), an osmotic minipump (2.5 μl/h for 28 days; Alza, Palo Alto, CA) was subcutaneously implanted in the females to deliver vehicle or nicotine tartrate (6 mg/kg per day) that produces nicotine blood levels approximately equivalent to those that occur in moderate to heavy smokers (45). Ten days after the implantation, each female rat was placed in a breeding cage with a male rat for up to 4 days. Those with vaginal plugs were considered pregnant and separated from the male. On the seventh day of gestation, the minipump was replaced with a new one filled accordingly with vehicle or nicotine. No more than three male pups from each litter with similar overall litter size were used in each study to minimize the possible effect of genetic difference between litters on the results. Males at postnatal day 12 to day 14 (P12-14) were chosen in this study because males are much more vulnerable than females in human sudden infant death syndrome (SIDS) (1), and brain development of pups at this period is equivalent to newborn infants at 2–4 mo (23). Pups from vehicle- and nicotine-treated dams were grouped as control (Ctrl) and PNE and randomly assigned to the following studies (also see Table 1).
Table 1.
Numbers of dams and their pups used in this study
| Pup # |
||||||||
|---|---|---|---|---|---|---|---|---|
| III |
||||||||
| Study Series | I | II | a | b | IV | V | VI | Dams # |
| Ctrl | 7 | 6 | 9 | 8 | 7 | 5 | 8 | 17 |
| PNE | 7 | 6 | 10 | 8 | 7 | 5 | 8 | 19 |
| Subtotal | 14 | 12 | 35 | 14 | 10 | 16 | ||
| Total | 101 | 36 | ||||||
I -VI represent 6 Study Series, and a and b represent the experimental subset in each Study Series. No more than 3 male pups from each litter were used to minimize the possible effect of genetic difference among litters on the results. 13 adult males were used for breeding. Ctrl, control; PNE, prenatal nicotinic exposure.
Experimental Protocols
Study series I.
Study series I was designed to examine whether PNE was able to change baseline-specific airway resistance (sRaw) and airway responsiveness because the airway hyperreactivity has been observed in mice with maternal cigarette-smoking exposure (57). The pups (n = 7 for Ctrl and PNE) were placed in a double-chambered plethysmograph (Buxco Electronics, Wilmington, NC). After stabilization, they were exposed to aerosolized vehicle and then methacholine (Sigma, St. Louis, MO) solutions in a dose-increasing manner (3.125, 6.250, 12.500, and 25.000 mg/ml) as previously reported (51).
Study series II.
Study series II was conducted to test whether the PNE-induced apnea and ventilatory arrest were of central origin or airway obstruction. To this end, both diaphragm electromyography (EMGdi) and ventilation were recorded in conscious Ctrl and PNE pups (n = 6 per group) previously instrumented with diaphragm electrodes. In a whole-body unrestrained plethysmograph chamber (PLY3211; Buxco Electronics), the animals were exposed to hypoxia (5% O2 balance with N2) for up to 60 min as reported before (68).
Study series III.
Study series III contained two sets of experiments. We examined whether PNE was able to augment the apneic response to right atrial injection of CAP (0.5 μg/kg) in anesthetized and spontaneously breathing pups (n = 9 and 10 for Ctrl and PNE pups). Because PNE prolonged the PCF-mediated apnea in our pilot study, this result encouraged us to further verify whether PNE was capable of facilitating pulmonary sensory C-neural response to CAP without change in baseline activity. Pulmonary C-neural activity in nodose ganglia was extracellularly recorded in anesthetized and paralyzed preparation (n = 8 in each group) before and during CAP injected into the right atrium.
Study series IV.
Study series IV was carried out to define whether PNE was capable of increasing TRPV1 gene and protein expression in the N/J ganglia by real-time PCR and Western blot, respectively. The pups (n = 7 in each group) were euthanized, and the N/J ganglia were bilaterally collected.
Study series V.
Study series V was designed to define PNE impact on the density of tracheal and bronchial SP-IR fibers. Because sensory C-fibers spread sparsely in the lungs, it is difficult to compare the density (56); here, we focused on comparison of tracheal and bronchial SP-IR fibers using immunocytochemistry in both groups (n = 5 in each group). Protein gene product (PGP)9.5 immunocytochemistry has been extensively used to mark nerve fibers. To estimate the effect of PNE on overall airway nerve fibers, PGP9.5-immunoreactive (PGP9.5-IR) fibers were colabeled with SP in two of the five PNE and Ctrl pups.
Study series VI.
Study series VI was performed to explore PNE effects on pulmonary NGF/BDNF and their receptors in N/J ganglia neurons. We detected NGF and BDNF protein expression in the lungs (ELISA) and their receptors (TrkB and TrkA/p75NTR gene) in the N/J ganglia (real-time PCR) in eight Ctrl and PNE pups.
General Animal Preparation
Conscious animals were used to detect sRaw, ventilation, and EMGdi activity after habituation to the chambers, whereas anesthetized pups (urethane, 1,200 mg/kg ip) were utilized to record the PCF-mediated apnea. Supplemental doses of anesthetics (120–240 mg/kg, urethane, iv) were provided as needed to suppress corneal and withdrawal reflexes. As previously reported (55), under adequate anesthesia, the trachea was cannulated and connected to a pneumotachograph (Frank's Manufacturing, Albuquerque, NM) to record airflow. The animals were exposed to a gas mixture of 30% oxygen in nitrogen throughout the experiment except exposure to hypoxia. The right femoral artery and jugular vein were cannulated for monitoring and recording arterial blood pressure (ABP)/heart rate (HR) and delivering drug into the pulmonary circulation, respectively. The core temperature of the animal was monitored with a rectal probe and maintained at ∼37.5°C with a heating pad and radiant heat. In 16 anesthetized animals, intravenous infusion of pancuronium (0.1–0.3 mg/kg for induction and 0.1 mg/kg per h for maintenance) were performed followed by artificial ventilation. During paralysis, the level of anesthesia was closely monitored by testing the responses of ABP and HR to paw pinch. An appropriate supplemental dose of the anesthetic was given once any obvious change (i.e., >10 mmHg increase in blood pressure or >20 beats/min increase in HR) was observed without or with paw pinch. A carbon dioxide analyzer (MicroCapStar end-tidal carbon dioxide analyzer, Model 15–10000; CWE, Ardmore, PA) was connected to a side-port of the tracheal cannula to monitor end-tidal pressure of carbon dioxide (PetCO2). With the ventilator, tidal volume (VT) was maintained at 10 ml/kg, and frequency varied to keep PetCO2 at ∼40 mmHg. Vagi were carefully isolated.
Experimental Approaches
sRaw responses to methacholine challenge.
sRaw was measured by using the two-chambered body plethysmograph system. Each pup was exposed to aerosols of methacholine for 1 min in a concentration-increasing manner (0.000, 3.125, 6.250, 12.500, and 25.000 mg/ml in isotonic saline) with a 5-min interval.
EMGdi and ventilation.
The pups were instrumented with a pair of electrodes (Cooner Wire Specialty, Chatsworth, CA) on the costal abdominal muscles via a right subcostal incision under adequate anesthesia with 3–5% isofluorane (47). Two hours later, the animal was exposed to hypoxia (5% O2 balance with N2) for up to 60 min in the whole-body plethysmograph with EMGdi leads exited out of the chamber and connected to the amplifiers. The temperature inside the chamber was maintained at ∼30.0°C (6, 35) with the animal body temperature maintained at ∼36.5°C.
PCF-mediated apneic response.
The catheter inserted into the right jugular vein was slowly loaded with CAP (0.5 μg) with a volume of 20 μl, and then the solution was quickly flushed into the right atrium with 50 μl saline. Vehicle was administered either before or after CAP injection with a 5-min interval between the two injections in each animal.
Extracellular recording of neurons within the nodose ganglion.
Briefly (65), after the right nodose ganglion was carefully isolated, the right midcervical vagus nerve 5 mm distal to the ganglion was attached to a stimulating electrode. A tungsten microelectrode (5 MΩ impedance; A-M Systems, Everett, WA) was progressively inserted into the ganglion, while the vagus nerve was electrically stimulated with 0.5-ms-duration square-wave pulses at an intensity of 0.5–1.0 mA. A reference electrode was placed on a skin incision near the recording electrode. The signals were amplified using a Grass P5 Series AC amplifier (Grass Instruments, Quincy, MA) with the filter band set at 1 Hz to 5 kHz. Pulmonary C-neurons were identified if the conduction velocity of the neurons responsive to the vagal electrical stimulation was <2 m/s and if neurons were activated by a bolus intra-atrial injection of CAP (18).
Quantitative PCR analysis of TRPV1, TrkA, TrkB, and p75NTR.
Total RNA in the N/J ganglia was isolated with RNeasy Mini Kit (Qiagen, Hilden, German), and <50 ng of RNA was converted to cDNA using Sensiscript RT Kit (Qiagen). Quantitative PCR was performed on ABI PRISM 7900 HT system (Applied Biosystems, Foster City, CA). Expression of TRPV1, TrkA, TrkB, and p75NTR gene was detected using TaqMan Gene Expression Assays from Life Technologies (Grand Island, NY); TRPV1: Rn00583117_m1, TrkA: Rn00572130_m1, TrkB: Rn01441749_m1, and p75NTR: Rn00561634_m1. Endogenous GAPDH (Rn01775763_g1) was also monitored for each assay. All qPCR results were represented as relative quantification.
Western blot analysis of TRPV1.
The N/J ganglia were pooled and homogenized with M2 buffer (20 mM Tris·HCl, pH 7.6, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM glycerophosphate, 1 mM sodium vanadate, and 1 μg/ml leupeptin). An equal amount of protein homogenates from each sample was run in 12% SDS-polyacrylamide gel electrophoresis, transferred to a PVDF membrane, and probed with goat anti-TRPV1 polyclonal antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. After incubation with horseradish peroxidase-conjugated anti-goat (1:2,000), the signals were detected by enhanced chemiluminescence according to the manuals (Millipore, Billerica, MA).
ELISA analysis of NGF and BDNF.
The protein levels of NGF and BDNF in lung homogenates were measured using the NGF Emax ImmunoAssay System (Promega, Madison, WI) according to the manufacturer's instructions. We prepared lung tissue homogenates as previously described (53).
Immunocytochemistry for detecting the density of airway PGP9.5-IR and SP-IR fibers.
After euthanasia, the animals were perfuse-fixed with 4% paraformaldehyde in PBS. Whole-mount axial airways were prepared for detecting the density of SP-IR and PGP9.5-IR fibers. The samples (up to the 3rd generation of bronchial) were properly prepared, permeabilized, immunodetected for SP and PGP9.5 with primary antibodies (mouse anti-PGP9.5 IgG mAb, Life Technologies; rabbit anti-SP pAb, Sigma), and visualized using Alexa Fluor 488/594 (Life Technologies). Omission of primary antibodies was included as negative control. Of each bronchial generation, at least two images were acquired using a ×10 water immersion objective at specified airway locations from the luminal surface with a digital camera (AxioCam HRm; Zeiss, Jena, Germany) connected to an epifluorescence microscope (Axioplan 2 FS, Zeiss). The density was quantified at the epithelial and submucosal layers of tracheal and bronchial levels at first, second, and third generations and expressed as the total length per unit area.
Data Acquisition and Statistical Analysis
Raw data of tidal volume (VT), respiratory frequency (fR), minute ventilation (VE), HR, mean ABP (MABP), sRaw, EMGdi, neural firing behavior, and temperature were recorded by PowerLab/8sp (model ML 785; AD Instruments, Colorado Springs, CO) and a computer with the LabChart Pro 7 software. An expiratory duration (tE) equal to or longer than 2 s was defined as an apnea (35, 58). The apnea concomitant with or without silencing of EMGdi activity was defined as a central (lack of inspiratory drive from the central nerve system) or obstructive apnea. All variables were expressed as absolute values with the exception that the apneic, HR, and MABP responses to CAP were presented as percentage. Group data were reported as means ± SE. Student's group t-test was used to analyze the significant differences between the two groups (Ctrl vs. PNE), whereas one- and two-way ANOVA with repeated measures was used to analyze the significant differences among the evoked response to CAP and different doses of methacholine in the two groups. If an overall test was significant, Tukey's test was utilized for specific comparisons between individual groups. P values <0.05 were considered significant.
RESULTS
PNE Has Little Effects on Behaviors of Pups
The pregnant rats undergoing PNE showed no discernible behavior abnormalities, such as agitation, loss of appetite, or shortness of breath. All of the pups in the two groups were delivered vaginally at full term of gestational D21 without dead fetuses found. There was no significant difference in birth numbers between Ctrl and PNE groups (12.3 ± 1.8 vs. 11.7 ± 1.4; P > 0.05). As presented in Table 2, PNE did not significantly alter the body weight and body temperature of the pups, and baseline VE, VT, and fR at conscious state.
Table 2.
PNE effect on BW, BT, and respiratory variables
| Ctrl (n = 15) | PNE (n = 15) | |
|---|---|---|
| BW, g | 32.4 ± 1.9 | 30.2 ± 3.5 |
| BT, °C | 36.7 ± 0.06 | 36.5 ± 0.05 |
| VE, ml/min | 26.3 ± 4.2 | 27.0 ± 3.6 |
| VT, ml | 0.18 ± 0.03 | 0.19 ± 0.03 |
| fR, breaths/min | 145 ± 13 | 142 ± 16 |
Values are means ± SE. BW, body weight; BT, body temperature; VE, minute ventilation; VT, tidal volume; fR, respiratory frequency. The data came from conscious pups in Studies Series I and II without implantation of diaphragm electromyography electrodes.
PNE Fails to Alter Baseline sRaw and Its Responsiveness
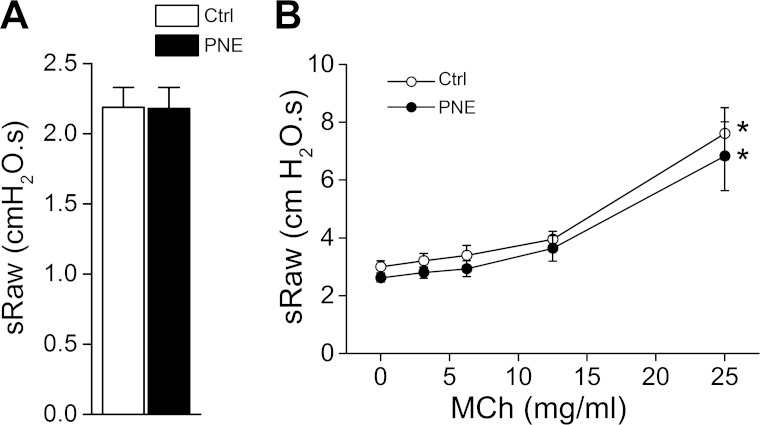
To ascertain the effects of PNE on airway resistance, we determined PNE effect on baseline sRaw and sRaw responses to increasing doses of methacholine in Ctrl and PNE pups. PNE did not significantly alter baseline sRaw values or sRaw responses to increasing doses of methacholine (Fig. 1, A and B). In addition, PNE also failed to change the threshold dose of methacholine (at 25 mg/ml) required for triggering a significant sRaw response.
Fig. 1.
Baseline airway resistance (sRaw) and its responses to methacholine (MCh) in control (Ctrl) and prenatal nicotinic exposure (PNE) pups. A: baseline values. B: airway response to saline (0) and MCh in a dose-increasing manner (3.125–25.0000 mg/ml); n = 7 in each group; means ± SE; *P < 0.05 compared with the responses to saline and lower doses of MCh.
Hypoxia Simultaneously Terminates Ventilation and EMGdi in PNE Pups
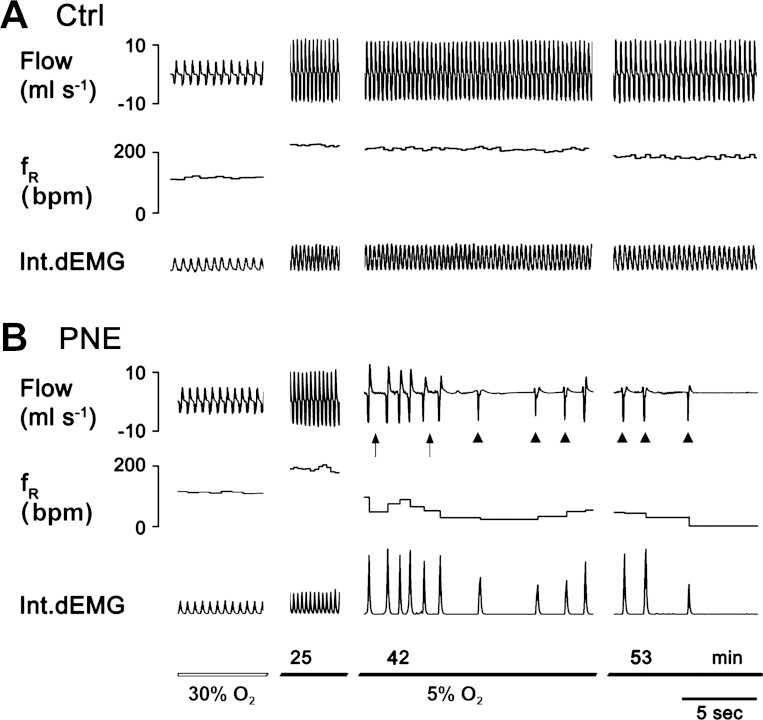
The lack of PNE effect on baseline sRaw and sRaw responsiveness described above does not necessarily mean that PNE is unable to induce airway obstruction during hypoxia-induced apnea. To confirm the central origin of the apnea, we compared the ventilatory and EMGdi responses to hypoxia in Ctrl and PNE pups. Figure 2 showed the typical ventilatory and EMGdi responses in a Ctrl and a PNE pup. Hypoxia increased ventilation at the beginning (25 min after hypoxia) and induced apnea (35 min after hypoxia), leading to lethal ventilatory arrest (50 min after hypoxia), which were identical to EMGdi activity in the PNE pups. In contrast, no apneic and ventilatory arrest responses were observed in the Ctrl pups. In other words, the apnea and lethal ventilatory arrests were of central origin attributable to silencing of both ventilation and EMGdi activity. Similarly, the group data showed that hypoxia did not induce any apneic and gasping response in all Ctrl pups, but the apneic (21 ± 5 episodes) and gasping responses (8 ± 4 episodes) occurred in all PNE pups. Importantly, all apneic and gasping responses noted in ventilation were corresponded in time to the EMGdi silencing. Similar to our previous report (68), PNE pups presented a significant reduction of hypoxic ventilatory response by 35% (baseline 24.5 ± 6 ml/min vs. hypoxia 55.6 ± 9 ml/min for Ctrl and baseline 25.1 ± 4 ml/min vs. hypoxia 45.9 ± 7 ml/min for PNE). A similar reduction (↓43%) in the responses of minute EMGdi activity [amplitude of integrated EMGdi (arbitrary unit) × fR] to hypoxia was also noted in PNE pups (baseline 8.2 ± 1.3 ml/min vs. hypoxia 14.0 ± 2.4 ml/min) compared with Ctrl pups (baseline 8.5 ± 2.0 ml/min vs. hypoxia 18.9 ± 2.8 ml/min).
Fig. 2.
The diaphragm electromyography (EMGdi) responses to hypoxia in a Ctrl and a PNE pup. A: hypoxia-induced ventilatory and EMGdi responses in a Ctrl pup without apnea presented within the time tested. B: hypoxia-induced apnea and subsequent lethal ventilatory arrest fully identical to the silencing of the EMGdi activity in a PNE pup. The traces from the top to bottom are airflow, respiratory frequency (fR), integrated EMGdi (Int.dEMG), and event marker(s)/duration of hypoxic exposure. Arrows and arrowheads point to the apnea and gasping in which the last gasp is lethal.
PNE Significantly Prolongs the PCF-Mediated Apnea
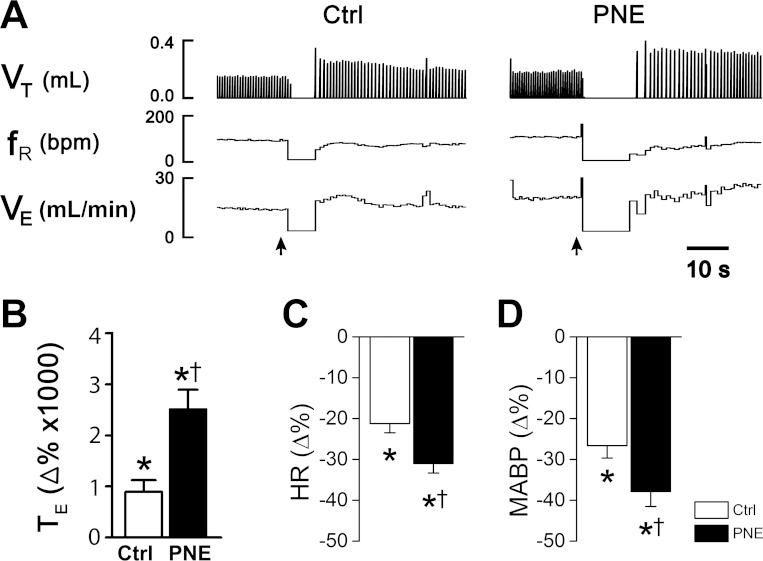
We compared the apneic responses to CAP in anesthetized and spontaneously breathing Ctrl and PNE pups. An example of experimental recordings is depicted in Fig. 3A. Apnea occurred immediately when CAP (0.5 μg/kg) was administered into the right atrium in a Ctrl pup. In sharp contrast, the same dose of CAP caused a much longer apnea in a PNE pup. The CAP-induced apnea usually occurred within one or two breaths after CAP bolus injection. Statistically, PNE, compared with Ctrl, did not significantly alter baseline tE (0.35 ± 0.06 s vs. 0.37 ± 0.05 s), HR (323.3 ± 14.1 beats/min vs. 326.7 ± 13.6 beats/min), and MABP (130.9 ± 10.2 mmHg vs. 133.4 ± 9.4 mmHg). However, the CAP-induced apnea was strikingly longer in PNE than Ctrl group (Fig. 3B); tE was prolonged by approximately ninefold in Ctrl pups in response to CAP (0.36 ± 0.04 s vs. 3.20 ± 0.34 s), but by 24-fold in PNE pups (0.38 ± 0.06 s vs. 9.17 ± 0.90 s). Vehicle was administered either before or after recovery from CAP-induced cardiorespiratory response in each animal, and it did not induce significant changes in tE in both groups (0.31 ± 0.04 s vs. 0.33 ± 0.03 s; P > 0.05). Additionally, CAP injection led to hypotension and bradycardia that were more evident in PNE than Ctrl pups (Fig. 3, C and D). Because our previous study has shown that the CAP-induced apneic response was highly reproducible in rat pups (55), we did not seek to confirm it again here (by injection of CAP twice in each animal).
Fig. 3.
Experimental recordings of the cardiorespiratory responses to bolus injection of capsaicin (CAP, 0.5 μg/kg iv) into the right atrium in anesthetized and spontaneously breathing rat pups. A: ventilatory responses to CAP in a Ctrl (left) and a PNE pup (right). In each panel, the traces from the top to bottom are tidal volume (VT), respiratory frequency (fR), minute ventilation (VE), and event markers (arrows) for CAP injection. B–D: group data showing the responses of expiratory duration tE (apnea), heart rate (HR), and mean arterial blood pressure (MABP) to CAP; n = 9 and 10 for Ctrl and PNE pups; means ± SE; *P < 0.01 compared with baseline (as 0) and †P < 0.01 between Ctrl and PNE pups.
PNE Increases Pulmonary Sensory C-Neural Response to Cap Without Effect on Baseline Activity
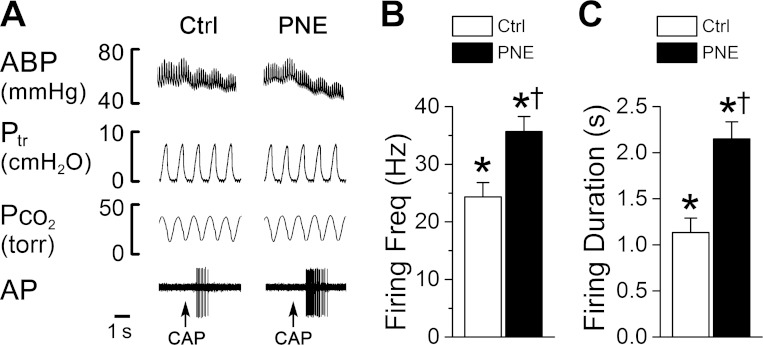
We found that the firing of the pulmonary C-neurons was usually irregular and had a relatively low frequency (0.35 ± 0.06 impulses/s) with conduction velocities (0.61 ± 0.04 m/s) similar to those found in previous reports (26). Of the 21 pulmonary C-neurons tested, CAP injection immediately increased PCF neural firing rate. The evoked firing rate and duration, as shown in Fig. 4, were significantly greater in PNE (12 cells) than Ctrl pups (9 cells). It is noteworthy that there was no difference in the baseline pulmonary C-neural firing between the two groups (0.32 ± 0.09 spikes/s vs. 0.34 ± 0.07 spikes/s, P > 0.05).
Fig. 4.
Effects of CAP (0.5 μg/kg iv) on pulmonary C-type neural firing bursts and duration in Ctrl and PNE pups. A: representative recordings showing the bursting response of pulmonary C-type neuron (the conduction velocity = 1.03 m/s) to intra-atrium injection of CAP in a Ctrl (left) and PNE pup (right). Traces from top to bottom are arterial blood pressure (ABP), tracheal pressure (Ptr), end-tidal fractional pressure of carbon dioxide (Pco2), and the firing activity of the cell (AP). B and C: group data showing that PNE increases CAP-induced C-neural firing rate and prolongs the firing duration. Neural firing was recorded in 12 and 9 C-type pulmonary neurons of PNE and Ctrl pups. Means ± SE; *P < 0.05 compared with baseline (as 0) and †P < 0.01 between Ctrl and PNE pups.
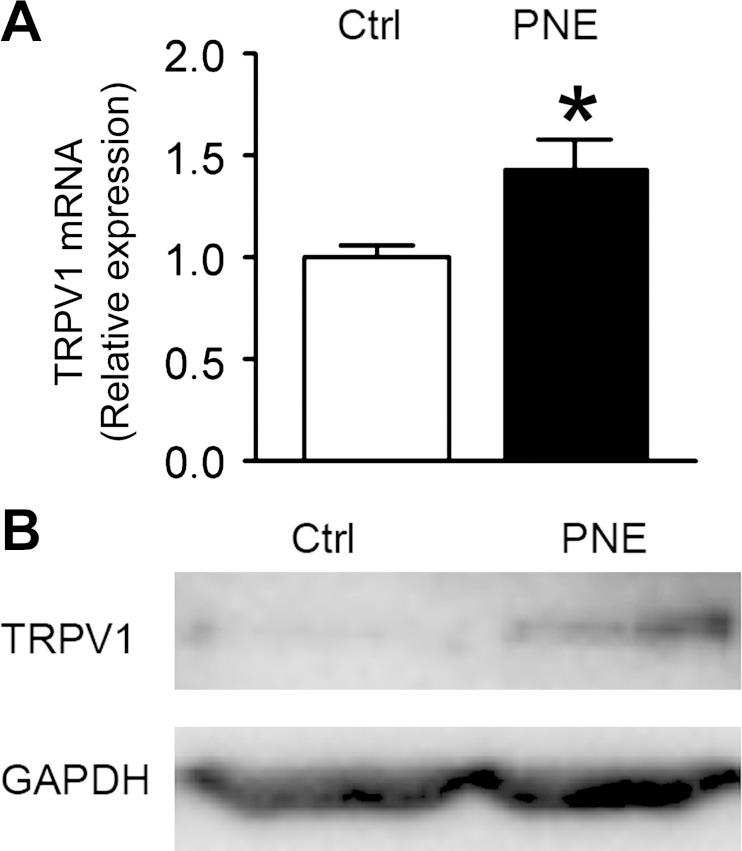
PNE Upregulates TRPV1 mRNA and Protein Expressions in N/J Ganglia Neurons
In this study, TRPV1 mRNA and protein expressions were detected in the N/J ganglia of both groups by employing real-time quantitative PCR analysis and Western blot, respectively. As illustrated in Fig. 5, PNE significantly enhanced TRPV1 gene and protein expressions in the N/J ganglia.
Fig. 5.
PNE impact on transient receptor potential cation channel subfamily V member 1 (TRPV1) expression in neurons of the nodose/jugular (N/J) ganglia. A: TRPV1 mRNA in the nodose ganglia detected by real-time PCR is significantly higher in PNE than Ctrl pups; n = 7 for each group; mean ± SE; *P < 0.05 compared with Ctrl. B: TRPV1 protein (100 kDa) was detected by Western blot in Ctrl and PNE samples from the pups' N/J ganglia with an equal amount of protein homogenates. GAPDH (37 kDa) functioned as a loading control.
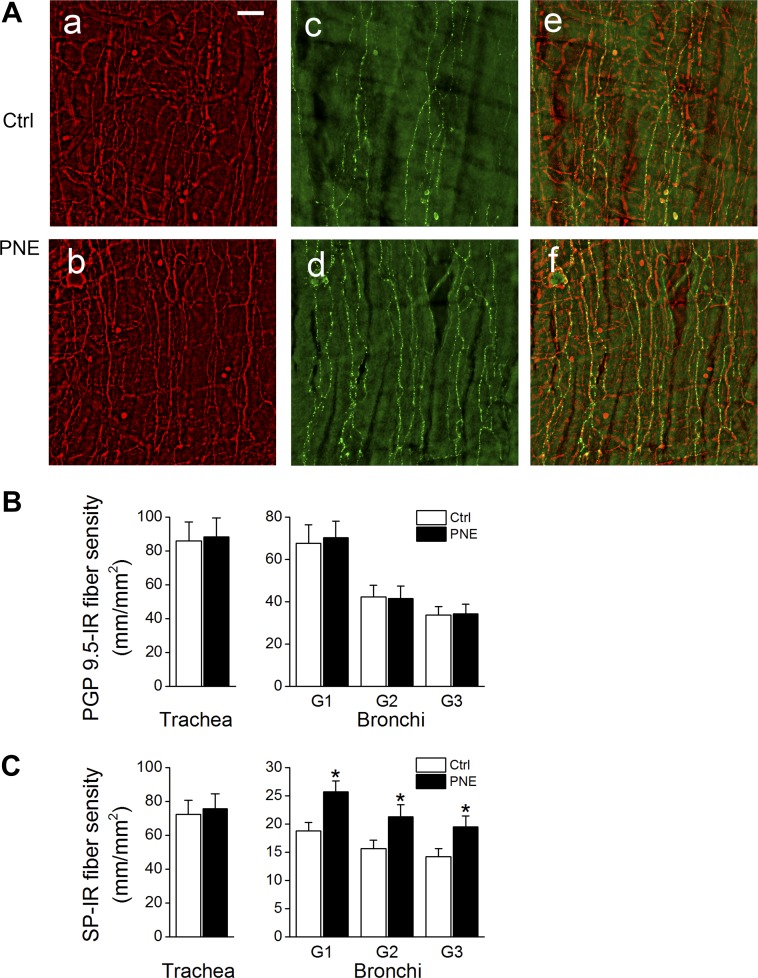
PNE Enhances the Density of Bronchial SP-IR Fibers
We compare the density of tracheal and bronchial PGP9.5-IR and SP-IR fibers between Ctrl and PNE pups. As exhibited in Fig. 6, the density of expression of tracheal PGP9.5-IR and SP-IR fibers was not markedly altered by PNE. However, the expression of SP-IR fibers, but not PGP9.5-IR fibers, in the first through third generations of bronchi was significantly increased by PNE (∼25% elevation).
Fig. 6.
PNE induced changes in the density of airway substance P-immunoreactive (SP-IR) fibers. A: typical micrographs showing protein gene product (PGP)9.5-IR (red), SP-IR (green) fibers, and their merged images in the epithelial and submucosal layers of first generation of bronchial wall in a Ctrl (top, a, c, e) and a PNE pup (bottom, b, d, f). The bar = 50 μm. B and C: group data of the PGP9.5-IR and SP-IR fiber densities of the trachea and bronchi (1st, 2nd, and 3rd generations, G1, G2, and G3), respectively; n = 5 for each group; means ± SE; *P < 0.05 between Ctrl and PNE pups.
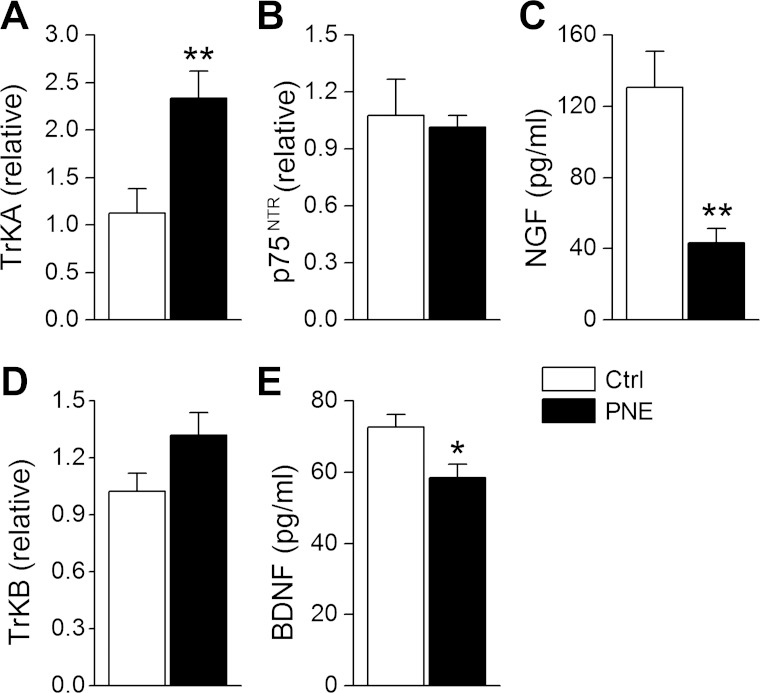
PNE Decreases Lung NGF and BDNF Proteins But Increases TrkA Gene in the N/J Ganglia
The concentrations of NGF and BDNF proteins in lung tissue homogenates were lower in PNE than Ctrl group as presented in Fig. 7, C and E. In contrast, PNE significantly upregulated gene expression of TrkA in N/J ganglia neurons without change in p75NTR and TrkB (Fig. 7, A, B, and D).
Fig. 7.
Effects on PNE on neurotrophic factors. A and D: levels of TrkB and TrkA mRNA in N/J ganglia neurons. C and E: concentrations of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) protein in lung homogenates. B: gene expression of p75NTR in N/J ganglia neurons; n = 8 in each group; means ± SE; *P < 0.05 and **P < 0.01 between Ctrl and PNE pups.
DISCUSSION
Studies have shown that PNE aggravates the apneic response to hypoxia, leading to a lethal ventilatory arrest in rat pups (38, 44, 64, 68). However, it is unknown whether these respiratory disorders result from central origin or airway obstruction. We found that the hypoxia-induced apnea and lethal ventilatory arrest are temporally identical to the silencing of EMGdi activities in PNE pups, suggesting the central origin of these respiratory disorders. In other words, the PNE-induced apnea and lethal ventilatory arrest during hypoxia result from the lack of inspiratory drive from the central nervous system. Maternal cigarette smoke could slightly but significantly increase airway response to aerosol methacholine in mice (43, 57) associated with an elevated expression of pulmonary muscarinic receptors (43). Thus we further clarified the PNE effect on airway response to methacholine. As the results show, PNE in rat pups failed to alter the airway responsiveness. This discrepancy may be due to the differences in the exposure (PNE vs. prenatal cigarette smoke) and species (rats vs. mice). In addition, a previous study indicated an increased density of PCF SP-IR by ovalbumin that promotes SP release in the bronchial lumen to induce bronchial hyperresponsiveness in mice (16). Our recent data showed that PNE, although it enhanced SP-IR density of PCFs, failed to significantly alter pulmonary SP release (66), which may also contribute to the lack of PNE impact on the airway response to methacholine in this study. Taken together, our results demonstrate a central origin of apnea and lethal ventilatory arrest observed in PNE pups during hypoxia without alteration in airway resistance.
Because of a critical role PCFs play in generating central apnea and PCF sensitization by exposure to acute nicotine (62) or maternal cigarette smoke (57), we subsequently asked whether PNE was capable of sensitizing PCFs. The present study, for the first time, indicates that PNE is able to augment the PCF-mediated cardiorespiratory responses via sensitizing PCFs because PNE did not alter baseline tE, but it remarkably augmented the PCF-mediated apnea, hypotension, and bradycardia. PNE-induced PCF sensitization was further confirmed by our observation that PNE profoundly increased pulmonary C-neural firing rate and burst duration without change in baseline PCF activity. In fact, PNE and maternal cigarette smoking have been reported to impair respiratory and cardiovascular function in rats (45, 68) and human infants (46). It is well documented that PCF sensitization by acute hypoxia (58) or by pulmonary infection (36, 39) could aggravate the PCF-mediated central apnea (7, 58) to a lethal degree. Thus our data, along with supersensitivity of PCFs and vagal C-fibers in early age (14, 33, 54), suggest that PCF sensitization in rat pups by PNE is likely, at least in part, responsible for the PNE-induced triggering of the apneic response to hypoxia.
The present study provides two lines of evidence promoting the concept that PNE leads to PCF sensitization presumably via raising PCF density and TRPV1 expression. First, PNE induces robust TRPV1 gene and protein expressions in the N/J ganglia. Second, PNE increases the density of bronchial SP-IR fibers in our study. It is worth noting that analysis of the entire ganglia in the present study is nonspecific because the N/J ganglion contains neurons innervating, not only the airway/lung, but also other organs (heart and gastrointestinal tract). In addition, the apneic and pulmonary C-type neural responses to right atrial injection of CAP are largely mediated by action of C-fibers in the lung, whereas the nerve fiber density labeled by SP-IR in this study was from bronchial C-fibers in the walls of the larger conducting airways. Despite the nonspecificity, we believe that PNE is capable of increasing TRPV1 expression and the density of C-fibers in the lungs because it has been established that the apneic response to right atrial bolus injection of CAP is predominantly mediated by action of PCF TRPV1 channel (15, 27). Moreover, the full dependency of pulmonary SP increase on PCFs in the mice exposed to cigarette smoke, of which nicotine is the major toxic component (60), supports a potential role of PNE in upregulating SP expression in PCFs. In the present study, we found that PNE did not strikingly change total density of bronchial nerve fibers labeled by PGP9.5. Two explanations may be accountable for the enhanced bronchial SP-IR density without changes in total nerve density. One is the shifting of some SP-negative bronchial C-fibers to SP-positive fibers after PNE. Recent reports have shown that some pulmonary Aδ-fibers that are normally SP negative become SP positive when animals are virally infected (48) or allergically sensitized (52). Thus another explanation is that this plasticity may also occur in bronchia and contributes to the increased density of bronchial SP-IR fibers after PNE.
The mechanisms underlying PNE sensitization of PCF response to activation of TRPV1 channel remain unclear. The PNE-induced sensitization of TRPV1 channel may result from changing the channel conformation because nicotine could promote the opening of TRPV1 channels (20, 25). It may also result from increasing the number of TRPV1 channels, as shown in this study. Interestingly, recent reports have shown that a sensitizing effect of nicotine on TRPV1 channel-mediated responses may be either dependent on or independent of activating neuronal nicotinic acetylcholine receptors (nAChRs). Nicotine induced a manifold increase in CAP-activated currents in rat trigeminal ganglion neurons via facilitating TRPV1 currents that are independent of the activation of neuronal nAChRs (30). On the other hand, nicotine increases the response of calcitonin gene-related peptide release to CAP in rat buccal mucosa, which is blocked by the nAChR antagonist (9). Therefore, further studies are warranted to define whether the PNE-induced PCF sensitization is nAChR dependent.
The upregulation of TRPV1 and SP expression in N/J ganglion may be related to the changes of neurotrophin induced by PNE. Several previous studies have determined that NGF, via binding to TrkA (21, 34), upregulates TRPV1 (2, 63) and SP expression (29) in primary sensory fibers. Moreover, NGF has been suggested to be involved in increasing the density of sensory fibers innervating airway in mice after prenatal exposure to smoke (57) and in guinea pigs sensitized with ovalbumin (8). We found that PNE decreased NGF but increased N/J neural TrkA gene with p75NTR unchanged. We speculate that the increased TrkA may overcome the NGF decrease to contribute to the overexpression of TRPV1 in N/J ganglia neurons and SP in bronchi sensory fibers. Nodose ganglion neurons also express TrkB (28), and its activation by BDNF is involved in promoting neural myelination of peripheral nerve fibers (11, 31), especially maturation of respiratory-related neurons in the nodose ganglion (22). Thus we also tested PNE effects on BDNF expression in lungs and TrkB gene expression in the N/J ganglia. As the results show, PNE markedly reduced BDNF without a significant change in TrkB. Considering that a newborn is at a critical period for vagal fiber development (37), we believe that the lower BDNF expression in PNE pups may be involved in delaying the maturity of PCFs, reflected by overexpression of SP-IR. Actually, a higher density of bronchial SP-containing nerves has been observed in newborn infants compared with adults (17). Again, the limitation of these data was that they came from N/J ganglionic neurons but not pulmonary C-neurons.
In summary, PNE failed to alter baseline sRaw and airway responsiveness but simultaneously terminated ventilation and EMGdi during hypoxia in PNE pups, leading to the conclusion that the PNE-induced apnea is of central origin. This central apnea promoted by PNE seems to be related to PCF sensitization because PNE sensitized the PCF-mediated apnea associated with upregulation of bronchial SP-IR fibers and TRPV1 expression in N/J ganglia neurons. PNE decreased pulmonary NGF and BDNF and increased N/J ganglia neural TrkA mRNA, which may contribute to the PNE-induced PCF plasticity.
Perspective
Our major findings in the present study are that PNE promotes occurrence of central apnea during hypoxia and augments the PCF-mediated cardiorespiratory responses by PCF sensitization, presumably via upregulation of TRPV1 and overexpression of pulmonary sensory fibers. These findings allow us to better understand how PNE triggers the apneic response to hypoxia and are beneficial to extending PCF cardiorespiratory pathophysiology during development. More importantly, these findings are relevant to SIDS. Although the pathogenesis of SIDS remains unclear, recent opinion points to triple risk for SIDS: critical period (2–4 mo old), intrinsic factor (maternal cigarette smoking), and exogenous stressor (hypoxemia) (50). Our animal model mimics the key risks (but not all risks) for SIDS with some respiratory dysfunctions similar to SIDS victims. Similar to our finding, the central apnea (13, 49) and an overexpression of C-fibers have been observed in SIDS victims (4). Moreover, a depressed ventilatory response to hypoxia and/or hypercapnia has been reported in the animals with overexpression of C-fibers (32) and vagal (PCF) sensitization (3, 59, 61, 67). Although our PNE may not fully reflect the impacts of prenatal cigarette smoke exposure, our results strongly suggest that nicotine is a significant component responsible for the detrimental effects of prenatal cigarette smoke on cardiorespiratory functions. Taken together, our results confirm the feasibility of our animal model for studying SIDS and are conceptually important in gaining new insight into mechanisms underlying the genesis of SIDS. Further studies are necessary to define the causal role of PCF sensitization in generating the PNE-induced cardiorespiratory failure and elucidate the mechanisms underlying PCF sensitization/plasticity by PNE.
GRANTS
This work was supported by HL 107462.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.Z. and F.X. conception and design of research; J.Z., L.Z., and N.Z. performed experiments; J.Z., L.Z., N.Z., and F.X. analyzed data; J.Z., L.Z., N.Z., and F.X. interpreted results of experiments; J.Z., L.Z., and N.Z. prepared figures; J.Z., L.Z., N.Z., and F.X. drafted manuscript; J.Z., L.Z., N.Z., and F.X. edited and revised manuscript; F.X. approved final version of manuscript.
REFERENCES
- 1.Adams EJ, Chavez GF, Steen D, Shah R, Iyasu S, Krous HF. Changes in the epidemiologic profile of sudden infant death syndrome as rates decline among California infants: 1990–1995. Pediatrics 102: 1445–1451, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, Birch R, Anand P. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett 399: 51–56, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Balan K, Kc P, Mayer C, Wilson C, Miller M, Martin R. LPS-mediated inflammation alters neonatal central respiratory control. FASEB J 24: 613.612, 2010. [Google Scholar]
- 4.Becker LE, Zhang W, Pereyra PM. Delayed maturation of the vagus nerve in sudden infant death syndrome. Acta Neuropathol (Berl) 86: 617–622, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci 15: 4918–4926, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boychuk CR, Fuller DD, Hayward LF. Sex differences in heart rate variability during sleep following prenatal nicotine exposure in rat pups. Behav Brain Res 219: 82–91, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 8.de Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, Fischer A. Airway hyper-responsiveness in allergic asthma in guinea-pigs is mediated by nerve growth factor via the induction of substance P: a potential role for TrkA. Clin Exp Allergy 36: 1192–1200, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dussor GO, Leong AS, Gracia NB, Kilo S, Price TJ, Hargreaves KM, Flores CM. Potentiation of evoked calcitonin gene-related peptide release from oral mucosa: a potential basis for the pro-inflammatory effects of nicotine. Eur J Neurosci 18: 2515–2526, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381: 706–709, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Fu KY, Dai LG, Chiu IM, Chen JR, Hsu SH. Sciatic nerve regeneration by microporous nerve conduits seeded with glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor gene transfected neural stem cells. Artif Organs 35: 363–372, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Hafstrom O, Milerad J, Sundell HW. Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am J Respir Crit Care Med 166: 1544–1549, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Harper RM, Kinney HC, Fleming PJ, Thach BT. Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Respir Physiol 119: 123–132, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Hasan SU, Sarnat HB, Auer RN. Vagal nerve maturation in the fetal lamb: an ultrastructural and morphometric study. Anat Rec 237: 527–537, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Hazari MS, Rowan WH, Winsett DW, Ledbetter AD, Haykal-Coates N, Watkinson WP, Costa DL. Potentiation of pulmonary reflex response to capsaicin 24 h following whole-body acrolein exposure is mediated by TRPV1. Respir Physiol Neurobiol 160: 160–171, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Hens G, Raap U, Vanoirbeek J, Meyts I, Callebaut I, Verbinnen B, Vanaudenaerde BM, Cadot P, Nemery B, Bullens DM, Ceuppens JL, Hellings PW. Selective nasal allergen provocation induces substance P-mediated bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 44: 517–523, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Hislop AA, Wharton J, Allen KM, Polak JM, Haworth SG. Immunohistochemical localization of peptide-containing nerves in human airways: age-related changes. Am J Respir Cell Mol Biol 3: 191–198, 1990. [PubMed] [Google Scholar]
- 18.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol 283: L494–L502, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Hui K, Liu B, Qin F. Capsaicin activation of the pain receptor, VR1: multiple open states from both partial and full binding. Biophys J 84: 2957–2968, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashiba H, Noguchi K, Ueda Y, Senba E. Coexpression of Trk family members and low-affinity neurotrophin receptors in rat dorsal root ganglion neurons. Brain Res Mol Brain Res 30: 158–164, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Katz DM. Regulation of respiratory neuron development by neurotrophic and transcriptional signaling mechanisms. Respir Physiol Neurobiol 149: 99–109, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Klaus B. Neuromodulation of the perinatal respiratory network. Curr Neuropharmacol 2: 221–243, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Krous HF, Campbell GA, Fowler MW, Catron AC, Farber JP. Maternal nicotine administration and fetal brain stem damage: a rat model with implications for sudden infant death syndrome. Am J Obstet Gynecol 140: 743–746, 1981. [DOI] [PubMed] [Google Scholar]
- 25.Kwak J, Wang MH, Hwang SW, Kim TY, Lee SY, Oh U. Intracellular ATP increases capsaicin-activated channel activity by interacting with nucleotide-binding domains. J Neurosci 20: 8298–8304, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee LY, Morton RF. Pulmonary chemoreflex sensitivity is enhanced by prostaglandin E2 in anesthetized rats. J Appl Physiol 79: 1679–1686, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Lieu T, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol 300: L790–L798, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 337: 362–364, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Zhu W, Zhang ZS, Yang T, Grant A, Oxford G, Simon SA. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. J Neurophysiol 91: 1482–1491, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 6: e17899, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MJ, Haxhiu MA, Georgiadis P, Gudz TI, Kangas CD, Macklin WB. Proteolipid protein gene mutation induces altered ventilatory response to hypoxia in the myelin-deficient rat. J Neurosci 23: 2265–2273, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortola J. Respiratory Physiology of Newborn Mammals: A Comparative Perspective. Baltimore, MD: The Johns Hopkins University Press, 2001. [Google Scholar]
- 34.Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci 13: 4029–4041, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pendlebury JD, Wilson RJ, Bano S, Lumb KJ, Schneider JM, Hasan SU. Respiratory control in neonatal rats exposed to prenatal cigarette smoke. Am J Respir Crit Care Med 177: 1255–1261, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Peng W, Zhuang J, Harrod KS, Xu F. Respiratory syncytial virus infection in anesthetized weanling rather than adult rats prolongs the apneic responses to right atrial injection of capsaicin. J Appl Physiol 102: 2201–2206, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Pereyra PM, Zhang W, Schmidt M, Becker LE. Development of myelinated and unmyelinated fibers of human vagus nerve during the first year of life. J Neurol Sci 110: 107–113, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol 538: 957–973, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabogal C, Auais A, Napchan G, Mager E, Zhou BG, Suguihara C, Bancalari E, Piedimonte G. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 57: 819–825, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Sandberg K, Poole SD, Hamdan A, Arbogast P, Sundell HW. Altered lung development after prenatal nicotine exposure in young lambs. Pediatr Res 56: 432–439, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 103: 637–647, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med 164: 989–994, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Singh SP, Mishra NC, Rir-Sima-Ah J, Campen M, Kurup V, Razani-Boroujerdi S, Sopori ML. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol 183: 2115–2121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ. Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for sudden infant death syndrome. Brain Res Bull 38: 69–75, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Slotkin TA, Saleh JL, McCook EC, Seidler FJ. Impaired cardiac function during postnatal hypoxia in rats exposed to nicotine prenatally: implications for perinatal morbidity and mortality, and for sudden infant death syndrome. Teratology 55: 177–184, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Sovik S, Lossius K, Walloe L. Heart rate response to transient chemoreceptor stimulation in term infants is modified by exposure to maternal smoking. Pediatr Res 49: 558–565, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Sunderram J, Semmlow J, Thakker-Varia S, Bhaumik M, Hoang-Le O, Neubauer JA. Heme oxygenase-1-dependent central cardiorespiratory adaptations to chronic hypoxia in mice. Am J Physiol Regul Integr Comp Physiol 297: R300–R312, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Tan YR, Yang T, Liu SP, Xiang Y, Qu F, Liu HJ, Qin XQ. Pulmonary peptidergic innervation remodeling and development of airway hyperresponsiveness induced by RSV persistent infection. Peptides 29: 47–56, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Thach BT. The role of respiratory control disorders in SIDS. Respir Physiol Neurobiol 149: 343–353, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics 129: 630–638, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turi GJ, Ellis R, Wattie JN, Labiris NR, Inman MD. The effects of inhaled house dust mite on airway barrier function and sensitivity to inhaled methacholine in mice. Am J Physiol Lung Cell Mol Physiol 300: L185–L190, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Undem BJ, Hunter DD, Liu M, Haak-Frendscho M, Oakragly A, Fischer A. Allergen-induced sensory neuroplasticity in airways. Int Arch Allergy Immunol 118: 150–153, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Urrego F, Scuri M, Auais A, Mohtasham L, Piedimonte G. Combined effects of chronic nicotine and acute virus exposure on neurotrophin expression in rat lung. Pediatr Pulmonol 44: 1075–1084, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang R, Xu F. Postnatal development of right atrial injection of capsaicin-induced apneic response in rats. J Appl Physiol 101: 60–67, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Wang R, Xu F, Liu J. Prenatal hypoxia preconditioning improves hypoxic ventilatory response and reduces mortality in neonatal rats. J Perinat Med 36: 161–167, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Wong SS, Sun NN, Keith I, Kweon CB, Foster DE, Schauer JJ, Witten ML. Tachykinin substance P signaling involved in diesel exhaust-induced bronchopulmonary neurogenic inflammation in rats. Arch Toxicol 77: 638–650, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Wu ZX, Hunter DD, Kish VL, Benders KM, Batchelor TP, Dey RD. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ Health Perspect 117: 1434–1440, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu F, Gu QH, Zhou T, Lee LY. Acute hypoxia prolongs the apnea induced by right atrial injection of capsaicin. J Appl Physiol 94: 1446–1454, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Xu F, Zhuang J, Wang R, Seagrave JC, March TH. Blunted ventilatory response to hypoxia/hypercapnia in mice with cigarette smoke-induced emphysema. Respir Physiol Neurobiol 158: 5–13, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Xu F. Role of neurogenic substance P in overexpression of alveolar macrophages' neurokinin 1 receptor in mice exposed to cigarette smoke. Exp Lung Res 36: 243–254, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Xu J, Xu F, Wang R, Seagrave J, Lin Y, March TH. Cigarette smoke-induced hypercapnic emphysema in C3H mice is associated with increases of macrophage metalloelastase and substance P in the lungs. Exp Lung Res 33: 197–215, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Yang W, Zhang G, Gu Q, Lee LY. Calcium transient evoked by nicotine in isolated rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 292: L54–L61, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 24: 4211–4223, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Zhuang J, Zhang C, Xu F. Inhalation of isoflurane alters the pulmonary C-fiber-mediated respiratory responses in anesthetized rats. FASEB J 25: 1111–1119, 2011. [Google Scholar]
- 65.Zhang Z, Zhuang J, Zhang C, Xu F. Isoflurane depolarizes bronchopulmonary C neurons by inhibiting transient A-type and delayed rectifier potassium channels. Respir Physiol Neurobiol 186: 164–172, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Zhao L, Zang N, Zhu Q, Zhuang J, Lee LY, Xu F. Upregulation of substance P, neurokinin-1 receptor, TRPV1, and adenosine A1R in nodose ganglia of rat pups treated with prenatal nicotinic exposure. FASEB J 28: 713–715, 2014. [Google Scholar]
- 67.Zhuang J, Xu F. Aerosolized capsaicin (CAP) pretreatment prevents lipopolysaccharide (LPS)-induced inhibition of ventilatory responses to hypoxia and hypercapnia in rats. FASEB J 22: 1172, 2008. [Google Scholar]
- 68.Zhuang J, Zhao L, Xu F. Maternal nicotinic exposure produces a depressed hypoxic ventilatory response and subsequent death in postnatal rats. Physiol Rep 2: 1–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]