Abstract
Cascade reactions initiated by radical addition to alkynes are synthetically very attractive because they enable access to highly complex molecular skeletons in only few synthetic steps under usually mild conditions. Here we report a general radical cascade reaction of alkynes, N-fluoroarylsulfonimides and alcohols, enabling the efficient synthesis of important α-amino-α-aryl ketones from readily available starting materials via a single operation. During this process, the highly regioselective nitrogen-centred radical addition to internal and terminal alkynes generating vinyl radicals and the next explicit migration of aryl group from the nitrogen source lead the following efficient desulfonylation, oxygenation, and semi-pinacol rearrangement. In addition, the semi-pinacol rearrangement precursors, α-alkyloxyl-α,α-diaryl imines, could also be efficiently obtained under milder conditions. This methodology might open a new entry for designing intermolecular radical cascade reaction of alkynes.
 Cascade and multi-component reactions allow highly functionalized molecules to be built from simpler precursors. Here, the authors report a three-component coupling giving α-amino-α-aryl ketones, initiated by the regioselective addition of nitrogen radicals to alkynes.
Cascade and multi-component reactions allow highly functionalized molecules to be built from simpler precursors. Here, the authors report a three-component coupling giving α-amino-α-aryl ketones, initiated by the regioselective addition of nitrogen radicals to alkynes.
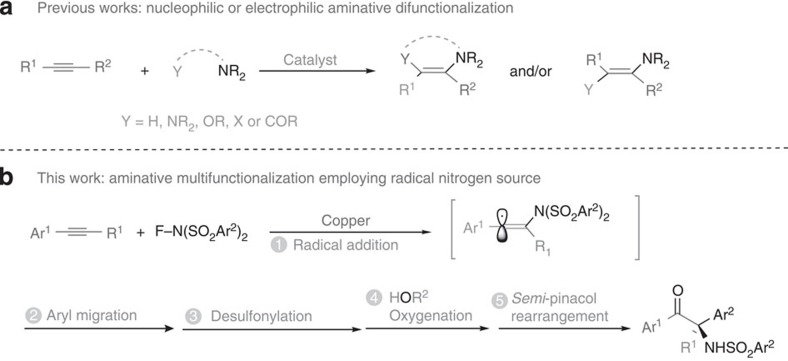
The regioselective construction of C–N bond under mild conditions remains an attractive research field due to the ubiquitous presence of amines in both naturally occurring and synthetic compounds, which manifest high levels of biological activity1,2. Alkyne functionalization, the addition of functional groups across a triple bond, exemplifies a class of reactions with significant synthetic potential. Accordingly, direct amination reaction of simple alkynes involving general intermolecular C–N bond construction step, such as hydroamiantion3,4,5,6, diamination7,8, aminooxygenation9,10,11,12, aminohalogenation13,14 and aminoacylation15 have been successfully developed, during which nucleophilic amination was usually involved with a few strategies employing electrophilic nitrogen sources (Fig. 1a). Cascade reactions initiated by radical addition to alkynes are synthetically very attractive because they allow access to highly complex molecular skeletons in only few synthetic steps under usually mild conditions, enabling them to exhibit high functional group compatiblity16. Although intermolecular radical addition to alkynes generating reactive vinyl radicals to perform intramolecular cascade reactions have been well established, their intermolecular multi-component equivalents remain a formidable challenge (vide infra). It is thus not surprising in that light that even simple addition reactions of nitrogen-centred radicals to alkynes are very rare17,18. In fact, compared with the well-established nucleophilic and electrophilic amination reaction, the construction of C–N bonds based on nitrogen-centred radicals have not received sufficient attention. The highly reactive vinyl radical generated by the addition of nitrogen-centred radical to alkynes offers a unique platform for radical-based processes mechanistically distinct from ionic pathways. We anticipate that this novel bond-forming strategy could be harnessed for facile construction of otherwise challenging nitrogen containing molecular architectures with traditional methodologies.
Figure 1. Aminative functionalization of alkynes.
(a) Nuclephilic or electrophilic aminative difunctionalization. (b) Radical cascade aminative multifunctionalization.
Challenges for the development of general cascade reactions initiated by nitrogen-centred radical addition to alkynes mainly resulted from two reasons: (1) the usually harsh conditions for the generation of nitrogen-centred radicals and their leading propensity for hydrogen abstraction or engaging in other degradation pathway; (2) the lack of a general intramolecular trapping manner to transfer the highly reactive incipient vinyl radical for further intermolecular cascade design. Recently, we developed copper-catalysed aminocyanation, diamination and aminoflurination reaction of alkenes via the efficient generation of nitrogen-centred radical from N-fluorobenzenesulfonimide (NFSI) under mild conditions19,20. As part of our continuing interest in employing NFSI as efficient amination nitrogen source21,22,23,24, in this article, a novel aminative multifunctionalization cascade reaction of alkyne with N-fluoroarylsulfonimide (as both nitrogen and aryl source) and alcohol (as oxygen source) was developed. Utilizing this simple transformation, α-amino-α-aryl ketones could be efficiently synthesized from both terminal and internal alkynes (Fig. 1b). α-Amino-α-aryl ketones, such as N-methylwelwitindolinone C isothiocyanate25, ketamine26 and prasugrel27 belong to an important class of biologically active natural products and pharmaceuticals. They are also useful precursors for the synthesis of heterocycles28,29,30,31 and 1,2-amino alcohols32,33. Recently, starting from readily available substrates, interesting methods for α-amino-α-aryl ketones such as cross-aza benzoin reaction of aldehydes with aryl imines34,35,36,37,38 and acyloin-type cross-coupling of aryl imines with nitriles39 were developed. Although significant progress has been made in the formation of C–N40,41,42,43 and C–C(aryl)44,45,46,47,48 bonds at α-position of the carbonyl group, it is a great challenge to simultaneously form C–N and C–C(aryl) bonds especially for the construction of quaternary carbon centres.
Herein, we report a cascade reaction that offers highly efficient construction of α-amino-α-aryl ketones starting from readily available alkynes, N-fluoroarylsulfonimides and alcohols via a highly efficient sequential regioselective nitrogen-centred radical addition to alkyne/aryl migration/desulfonylation/oxygenation/semi-pinacol rearrangement process (Fig. 1b). In addition, the semi-pinacol rearrangement precursors, α-alkyloxyl-α,α-diaryl imines, could also be efficiently obtained under milder conditions.
Results
Optimization for the sythesis of α-amino-α-aryl ketones
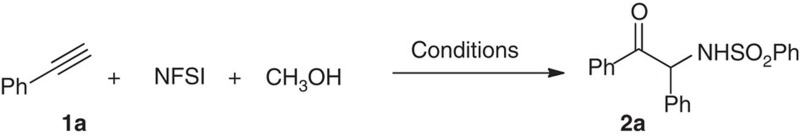
On the basis of previous reports developed by us19,20 and others49,50,51, we sought to use NFSI as both nitrogen source and aryl source to investigate aminative multifunctionalization of alkynes. Our investigation commenced with the reaction of phenylacetylene (1a, 0.5 mmol) with NFSI (0.75 mmol, 1.5 equiv.) in the presence of Cu(OTf)2 (10 mol %) at 90 °C in commercially available CH3CN (2 ml) under N2 atmosphere, α-amino-α-aryl ketone 2a was obtained in 30% yield after 8 h. However, when dry CH3CN was used, no reaction occurred. Therefore, water (1.5 mmol, 3 equiv.) was added to the reaction system, α-amino-α-aryl ketone 2a was obtained in 37% yield. In this reaction, C–N, C–C(aryl) and C=O bonds were simultaneously introduced to alkyne 1a. Delightfully, the readily available CH3OH was viable and furnished 2a in 58% yield (Table 1, entry 1). With pyridine-N-oxide or CH3COOH as oxygen source, no 2a was obtained. So the reaction of 1a with NFSI and CH3OH was used as the model to optimize the reaction conditions. As shown in Table 1, other catalysts, such as CuCl, Fe(OTf)2, Zn(OTf)2 and Sc(OTf)3 could catalyse the reaction, but no improved result was obtained (Table 1, entry 2–5). A decrease in the temperature from 90 to 70 °C afforded 2a in 54% yield (Table 1, entry 6). Further lowering the temperature to 50 °C resulted in sluggish reaction and only a trace amount of 2a was observed (Table 1, entry 7). Screening of solvents (Table 1, entries 8–10) identified CH3CN as the solvent of choice. Finally, a satisfactory yield of 78% was achieved when CF3COOH was employed as additive (Table 1, entries 11–14). Considering the number of steps involved in this process, this overall yield indicates of high efficiency of this radical involved cascade. Interestingly, the reaction could also proceed at 130 °C without catalyst to provide 2a in 32% yield (Table 1, entry 16).
Table 1. Optimization of the reaction conditions.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst | Additive | Solvent | Temperature (oC) | Time (h) | Yield (%) |
| 1 | Cu(OTf)2 | None | CH3CN | 90 | 5 | 58 |
| 2 | CuCl | None | CH3CN | 90 | 5 | 40 |
| 3 | Fe(OTf)2 | None | CH3CN | 90 | 7 | 50 |
| 4 | Zn(OTf)2 | None | CH3CN | 90 | 20 | 47 |
| 5 | Sc(OTf)3 | None | CH3CN | 90 | 20 | 44 |
| 6 | Cu(OTf)2 | None | CH3CN | 70 | 5 | 54 |
| 7 | Cu(OTf)2 | None | CH3CN | 50 | 24 | Trace |
| 8 | Cu(OTf)2 | None | DCM | 70 | 24 | 34 |
| 9 | Cu(OTf)2 | None | EtOAc | 70 | 24 | NR* |
| 10 | Cu(OTf)2 | None | THF | 70 | 24 | —† |
| 11 | Cu(OTf)2 | C6H5COOH | CH3CN | 70 | 4 | 60 |
| 12 | Cu(OTf)2 | CH3COOH | CH3CN | 70 | 3 | 61 |
| 13 | Cu(OTf)2 | CF3SO3H | CH3CN | 70 | 4 | 40 |
| 14 | Cu(OTf)2 | CF3COOH | CH3CN | 70 | 5 | 78 |
| 15 | None | None | CH3CN | 70 | 24 | NR* |
| 16 | None | None | CH3CN | 130 | 10 | 32 |
Reaction conditions: 1a (0.5 mmol), NFSI (1.5 equiv., 0.75 mmol), CH3OH (3 equiv., 1.5 mmol), catalysts (10 mol %), additives (1 equiv., 0.5 mmol), anhydrous solvents (2 ml), N2 atmosphere. Isolated yield.
*NR, no reaction.
†HN(SO2Ph)2 was identified.
Scope of terminal alkyne and N-fluoroarylsulfonimide substrates
With the optimized conditions at hand (Table 1, entry 14), the scope of aminative multifunctionalization of terminal alkynes was investigated. The tested phenylethetylene derivatives 1 smoothly reacted with NFSI and CH3OH to afford the corresponding α-amino-α-aryl ketones 2 or 3 in 48–78% yields (Table 2). For α-amino-α-aryl ketones 2, phenyl group from NFSI connected to the terminal carbon of alkynes and for α-amino-α-aryl ketones 3, aryl group from alkynes 1 connected to the terminal carbon of alkynes. For alkynes 1b–1f which bear ortho-substitutions, 2b–2f became the major products with the less sterically hindered phenyl group selectively migrated, forming C−C (aryl) bonds. Alkynes 1g–1o with electron donating or withdrawing groups afforded major products 2g–2i and 3j–3o in which the comparatively electron-rich aromatic ring migrated to form C−C (aryl) bonds. In addition, NFR1 (N-fluoro-4-methyl-N-tosylbenzenesulfonamide) was used instead of NFSI to further explore the scope of this alkyne aminative multifunctionalization. As expected, the reaction of 1 with NFR1 and CH3OH proceeded smoothly and provided 4a–h and 5a–h (with 4a–h as major products) in 48–84% yield (Table 3). Similar electronic and steric effects as using NFSI were observed. Trifluoromethyl and cyano groups were compatible and provided the corresponding α-amino-α-aryl ketones 4g (81%) and 4h (48%). However, substrates with strong electron-donating groups on the aromatic ring, such as 1-ethynyl-4-methoxybenzene and 1-ethynyl-3-methoxybenzene, were not effective. In addition, reactions between 1-(tert-butyl)-4-ethynylbenzene and some other NFSI derivatives were also explored to extend scope and investigate electronic effect of aryl part of NFSI derivatives. For 4-chloro-N-((4-chlorophenyl)sulfonyl)-N-fluorobenzenesulfonamide (NFR2), 5i was obtained as a single isomer in yield of 46%. For N-fluoroarylsulfonimides 4-tert-butyl-N-fluoro-N-(phenylsulfonyl)benzenesulfonamide (NFR3) and 4-tert-butyl-N-(4-chlorophenylsulfonyl)-N-fluorobenzenesulfonamide (NFR4), the corresponding α-amino-α-aryl ketones were obtained in 72 and 67% yield, respectively. These results showed that the transformation was more efficient for electron-rich aromatic rings than electron-poor aromatic rings in NFSI derivatives. The halogen atom on the aromatic ring was tolerated in this process (2e–i, 4c–f, 5i, 5k), offering an opportunity for further elaboration.
Table 2. Aminative multifunctionalization of terminal alkynes with NFSI.
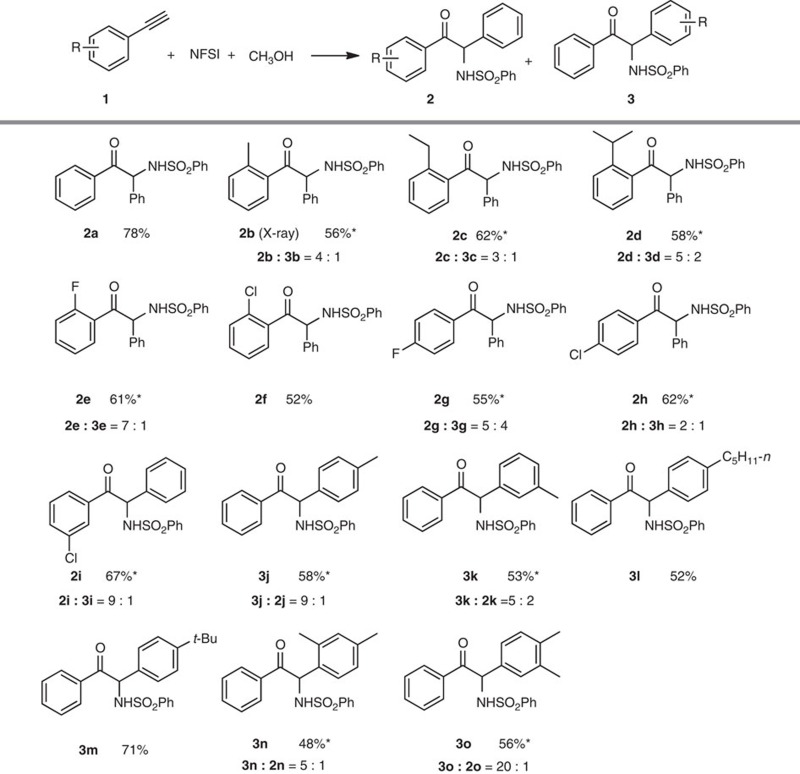 |
NFSI, N-fluorobenzenesulfonimide.
Reaction condition: 1 (0.5 mmol), NFSI (1.5 equiv., 0.75 mmol), CH3OH (3 equiv., 1.5 mmol), Cu(OTf)2 (10 mol %) and TFA (1.0 equiv., 0.5 mmol) in CH3CN (2 ml) at 70 °C under N2 atmosphere for 5 h. Isolated yield.
*Mixture of two isomers. The ratio was determined by 1H NMR analysis.
Table 3. Aminative multifunctionalization of terminal alkynes with NFR1–4.
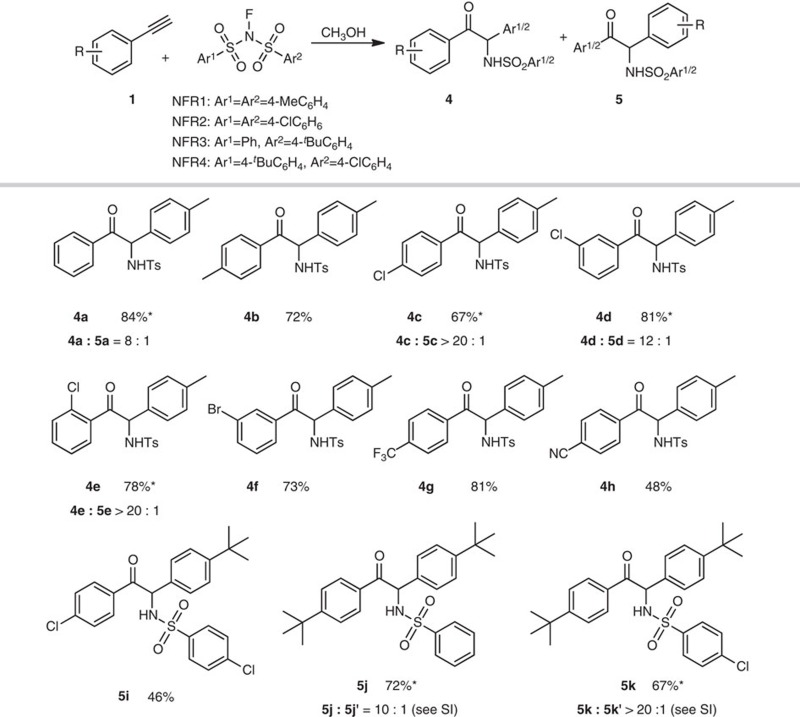 |
Reactions condition: 1 (0.2 mmol), NFR1–4 (1.5 equiv., 0.3 mmol), CH3OH (3 equiv., 0.6 mmol), Cu(OTf)2 (10 mol %) and TFA (1.0 equiv., 0.2 mmol) in CH3CN (2 ml) at 90 °C under N2 atmosphere for 8 h. Isolated yields.
*Mixture of two isomers. The ratio was determined by 1H NMR analysis.
Scope of internal alkyne substrates
To examine the generality of this alkyne aminative multifunctionalization, internal alkynes were subsequently examined. In the presence of 5 mol% CuCN, the reaction of pent-1-yn-1-ylbenzene (6a, 0.5 mmol), NFSI (1.5 equiv., 0.75 mmol) and i-PrOH (1.5 equiv., 0.75 mmol) in dichloromethane (DCM, 2 ml) was carried out at 70 °C under nitrogen atmosphere for 12 h. The expected α-amino-α-aryl ketone 8a with a quaternary carbon at α-position was afforded in 68% yield (Table 4). As shown in Table 4, an array of α-amino-α-aryl ketones 8 and/or 9 were obtained in yields ranging from 48 to 73%. Similarly, preferential migration of electron-rich aromatic substituent (aryl on the alkyne versus Ph from NFSI) was observed in the product distribution. It should be noted that this aminative multifunctionalization of internal alkynes directly lead to the skeleton of α-tertiary amine derivatives, which are widespread in various natural products and bioactive compounds52,53,54,55. Quaternary carbon centres with a nitrogen substituent have been successfully constructed through molecular rearrangement56. However, special structures of substrates were necessary. Therefore, the directly aminative multifunctionalization of alkynes could provide a new and facile way for α-tertiary amines. Recently, Murakami and co-workers57 reported an interesting Cu- and Rh-catalyzed aminative multifunctionalization of terminal alkynes to form α-amino-α-allyl ketones via α-imino metal carbene intermediate, during which C–C(allyl) bond formed through Claisen-type rearrangement. In their study, for internal alkynes, N-sulfonyl-1,2,3-triazoles needed to be pre-prepared.
Table 4. Aminative multifunctionalization of internal alkynes with NFSI.
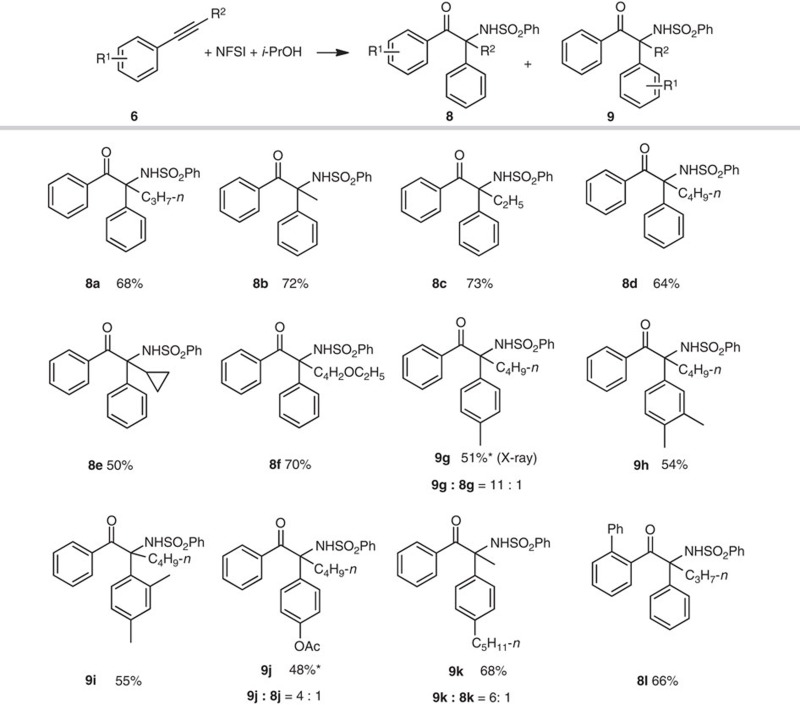 |
NFSI, N-fluorobenzenesulfonimide.
Reactions condition: 6 (0.5 mmol), NFSI (1.5 equiv., 0.75 mmol), i-PrOH (1.5 equiv., 0.75 mmol), CuCN (5 mol %) and ZnCl2 (2 mol %) in CH2Cl2 (2 ml) at 70 °C under N2 atmosphere for 12 h. Isolated yields.
*Mixture of two isomers. The ratio was determined by 1H NMR analysis.
Mechanism investigation
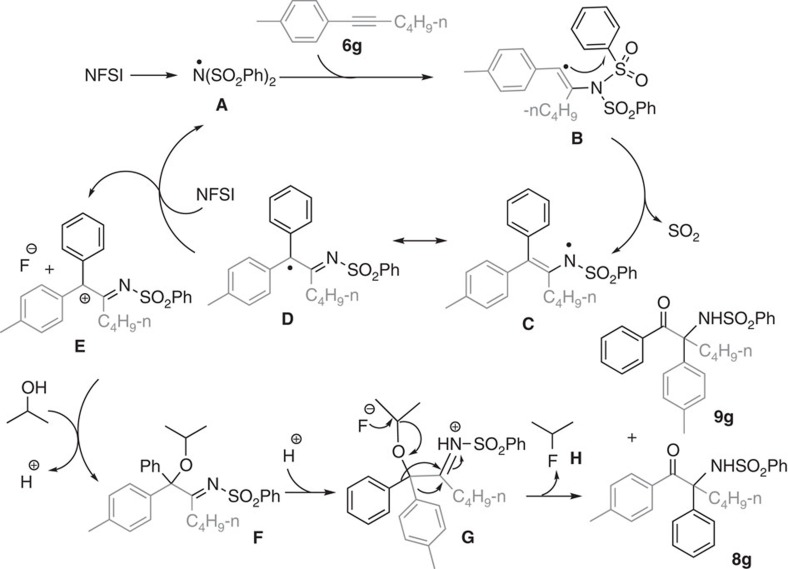
Radical scavengers were employed to probe the mechanism of the aminative multifunctionalization of alkynes. Formation of 2a was completely inhibited when 1 equivalent of 2,6-di-tert-butyl-4-methylphenol or 2,2,6,6-tetramethyl-1-piperidinyloxy was added to the reaction. For the reaction with 2,6-di-tert-butyl-4-methylphenol as radical scavenger, 26% benzylic C–H amination product was obtained. These results suggested a possible radical mechanism. In combination with our previous finding in amination19,20,21,22,23,24, we proposed a possible mechanism as depicted in Fig. 2. Initially, the in situ-generated nitrogen-centred radical A added to the triple bond of alkyne (for example 6g) regioselectively, providing a highly reactive vinyl radical B. Subsequently, sequential intramolecular 1,4-aryl migration via 5-ipso cyclization and desulfonylation would produce amidyl radical C58,59,60,61. This imidyl radical exists at an equilibrium with its resonance structure α-imino carbon radical D which could be stabilized by two aromatic rings and a C=N double bond. Then, the single-electron oxidation of intermediate D by NFSI generated a carbocation intermediate E and a nitrogen-centred radical A to continue the next catalytic cycle. The reaction between intermediate E and ROH provided α-alkyloxyl imine F. Finally, the protonation and semi-pinacol rearrangement of intermediate F furnished aminative multifunctionalization to provide isomer 8g and 9g. The ratio of 8g to 9g depended on electronic and steric effects of the corresponding aromatic substituents. As depicted in Tables 2, 3, 4, electron-rich and the sterically less-hindered aromatic rings are more prone to migrate, which is in consistency with the requirements of semi-pinacol rearrangement. It is noted that during this transformation, trapping of the incipient vinyl radical by aromatic ring from nitrogen source was a key step leading to intermolecular cascade process, which might provide a new entry to design radical addition initiated multi-component cascade reaction of alkynes.
Figure 2. Proposed mechanism.
Sequential regioselective nitrogen-centred radical addition to alkyne/aryl migration/desulfonylation/oxygenation/semi-pinacol rearrangement were involved.
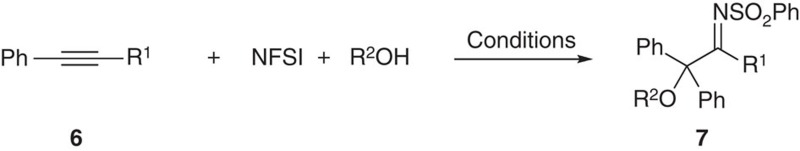
Since the above-mentioned mechanism invoked a semi-pinacol rearrangement from a relatively stable species α-alkyloxyl-α,α-diaryl imine F to the final product, we questioned if this species could be obtained separately with modification of reaction parameters. Recently, semi-pinacol rearrangement of α-hydroxy imines had been successfully applied in natural product as well as catalytic asymmetric syntheses62,63,64. To our delight, the reaction of pent-1-yn-1-ylbenzene (6a, 0.5 mmol), NFSI (1.0 mmol, 2.0 equiv) and propan-2-ol (1.5 mmol, 3.0 equiv.) in the presence of Cu(acac)2 (5 mol %) at 0 °C in dry CH3CN (2 mL) under N2 atmosphere was performed for 48 h, α-alkyloxyl-α,α-diaryl imine 7a was obtained in 71% yield. Besides the reaction temperature, catalyst played an important role in obtaining this product because no desired 7a was obtained without copper. As shown in Table 5, various alcohols could react with NFSI and alkynes to obtain the corresponding α-alkyloxyl-α,α-diaryl imine 7a–7j in 46–71% yields. It should be noted that diaryl-substituted alkynes are also effective. Starting from 1,2-diphenylethyne (6k), the corresponding α-alkyloxyl-α,α-diaryl imine was obtained in 66% under relatively higher temperature (Table 5, entry 11). Interestingly, for substrate 6l, nitrogen-centred radical highly regioselectively added to the alkyne carbon connected to the aromatic ring with strong electron-withdrawing NO2 group. From substrates 6m and 6n, regioisomer mixtures (7m:7m′=1.5:1, 7n:7n′=1:2) were obtained.
Table 5. Syntheses of α-alkyloxyl-α,α-diaryl imine.
 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2OH | Temperature (oC) | Product | Yield (%) |
| 1 | n-C3H7 | i-PrOH | 0 | 7a | 71 |
| 2 | Me | i-PrOH | 10 | 7b | 68 |
| 3 | n-C4H9 | i-PrOH | 0 | 7c | 63 |
| 4 | n-C3H7 | CH3OH | 10 | 7d | 52 |
| 5 | n-C3H7 | EtOH | 10 | 7e | 62 |
| 6 | n-C3H7 | Butan-1-ol | 10 | 7f | 58 |
| 7 | n-C3H7 | Butan-2-ol | 0 | 7g | 65 |
| 8 | n-C3H7 | Cyclohexanol | 10 | 7h | 70 |
| 9 | n-C3H7 | Prop-2-yn-1-ol | 25 | 7i | 54 |
| 10 | n-C3H7 | (E)-but-2-en-1-ol | 25 | 7j | 46 |
| 11* | Ph | CH3OH | 90 | 7k | 66 |
| 12* | 4-NO2C6H4 | CH3OH | 90 | 7l | 45 |
| 13* | 4-acetyl C6H4 | CH3OH | 90 | 7m:7m′=1.5:1 | 41† |
| 14* | 4-tBuC6H4 | CH3OH | 90 | 7n:7n′=1:2 | 54† |
Reactions condition: 6 (0.5 mmol), NFSI (2 equiv., 1.0 mmol), R2OH (3 equiv., 1.5 mmol), Cu(acac)2 (5 mol %) in CH3CN (2 ml) under N2 atmosphere for 48 h. Isolated yields.
*Reactions condition: 6 (0.5 mmol), NFSI (2 equiv., 1.0 mmol), CH3OH (3 equiv., 1.5 mmol), CuCN (5 mol %) in CH2Cl2 (2 ml) at 90 °C under N2 atmosphere for 48 h. Isolated yields.
†Mixture of two isomers. The ratio was determined by 1H NMR analysis.
Identification of intermediate F (Fig. 1) provided strong proof to the proposed mechanism. Therefore, further experiments for more mechanistic information were also carried out. The final α-amino-α-aryl ketone 8a could be obtained in 87% yield when heating 7a (0.3 mmol) at 70 °C in the presence of 0.3 mmol trifluoroacetic acid (TFA) in 2 ml DCM for 4 h, lending further support for 7 as the key intermediate in the novel aminative multifunctionalization of alkynes. However, under the same conditions but adding CuCN (5 mol %) instead of TFA, no reaction occured. Instead, when ZnCl2 (10 mol%) was added, 8a was isolated in 60% yield, which showed that ZnCl2 additive in Table 4 played an important role for the transformation from intermediate F (Fig. 2) to final α-amino-α-aryl ketones. When the semi-pinacol rearrangement of 7a was performed in the presence of HF acid (1 equiv., 40 wt % in water) instead of TFA, 8a could be obtained in 68% yield, along with identified side-product 2-fluoropropane (H, Fig. 2). Starting from 7l, the next semi-pinacol rearrangement process was not effective, which elucidated why transformation from diaryl-substituted alkynes to the desired α-amino-α-aryl ketones could not be realized in this study. Starting from 6a, when t-BuOH was employed instead of i-PrOH under otherwise same conditions described in Table 5, entry 9, a α-fluoro-α,α-diaryl imine could be obtained in 36% yield, which demonstrated the possible presence of intermediate E (Fig. 2).
In conclusion, an unprecedented cascade radical aminative multifunctionalization reaction of various aryl terminal and internal alkynes with N-fluoroarylsulfonimides and simple alcohols is developed. This methodology provides a new facile and straightforward way for both α-amino-α-aryl ketones and α-alkyloxyl-α,α-diaryl imines, especially for the construction of quaternary α-amino ketones, which might open a new entry for designing multi-component radical cascade reactions of alkynes. Further studies for the application of this transformation are ongoing in our laboratory.
Methods
General methods
For 1H, 19F and 13C NMR spectra of compounds in this manuscript, see Supplementary Figs 1–121. For details of the synthetic procedures, tables including detail experimental, see Supplementary Information.
Preparation of 2a
To a solution of the NFSI (0.75 mmol, 236.5 mg) in CH3CN (2.0 ml) was added the CH3OH (1.5 mmol, 61 μl), TFA (0.5 mmol, 37 μl), 1-Phenylethyne (1a, 0.5 mmol, 54 μl) and Cu(OTf)2 (0.05 mmol, 18.1 mg) in screw-cap test tube under N2 atmosphere. The test tube was then sealed off with a screw-cap and the reaction mixture was stirred at 70 °C for 5.0 h. After the reaction finished, the reaction mixture was cooled to room temperature and quenched by water. The mixture was extracted with CH2Cl2 (3 × 5.0 ml), the combined organic phases were dried over anhydrous Na2SO4 and the solvent was evaporated under vacuum. The residue was purified by column chromatography (petroleum ether/ethyl acetate (10:1 v/v)) to give the corresponding product 2a (136.9 mg, 78%).
Preparation of 8a
To a solution of the NFSI (0.75 mmol, 236.5 mg) in CH2Cl2 (2.0 ml) was added the isopropanol (0.75 mmol, 57 μl), but-1-yn-1-ylbenzene (6a, 0.5 mmol, 80 μl), ZnCl2 (0.01 mmol, 1.4 mg) and CuCN (0.025 mmol, 2.2 mg) in screw-cap test tube under N2 atmosphere. The test tube was then sealed off with a screw-cap and the reaction was stirred at 70 °C for 12.0 h. After the reaction finished, the reaction mixture was cooled to room temperature and quenched by water. The mixture was extracted with CH2Cl2 (3 × 5.0 ml), the combined organic phases were dried over anhydrous Na2SO4 and the solvent was evaporated under vacuum. The residue was purified by column chromatography (petroleum ether/ethyl acetate 10:1 (v/v)) to give the corresponding product 8a (133.7 mg, 68%).
Preparation of 7a
To a solution of the NFSI (1.0 mmol, 314.3 mg) in CH3CN (2.0 ml) was added the isopropanol (1.5 mmol, 114 μl), but-1-yn-1-ylbenzene (6a, 0.5 mmol, 80 μl) and Cu(acac)2 (0.025 mmol, 6.5 mg) in screw-cap test tube under N2 atmosphere. The test tube was then sealed off with a screw-cap and the reaction was stirred at 0 °C for 48.0 h. After the reaction finished, the reaction mixture was quenched by water. The mixture was extracted with CH2Cl2 (3 × 5.0 ml), the combined organic phases were dried over anhydrous Na2SO4 and the solvent was evaporated under vacuum. The residue was purified by column chromatography (petroleum ether/diethyl ether (25:1 v/v)) to give the corresponding product 7a (154.5 mg, 71%).
Preparation of 7k
To a solution of NFSI (1.0 mmol, 315.3 mg) in CH2Cl2 (2.0 ml) was added methanol (1.5 mmol, 61 μl), 1,2-diphenylethyne (6k, 0.5 mmol, 89 mg) and CuCN (0.025 mmol, 2.2 mg) in screw-cap test tube under N2 atmosphere. The test tube was then sealed off with a screw-cap and the reaction was stirred for the 48.0 h at 90 °C. After the reaction finished, the reaction mixture was cooled to room temperature and quenched by water. The mixture was extracted with CH2Cl2 (3 × 5.0 ml), the combined organic phases were dried over anhydrous Na2SO4 and the solvent was evaporated under vacuum. The residue was purified by column chromatography (petroleum ether/ethyl acetate 20:1 (v/v)) to give the corresponding product 7k (145.6 mg, 68%).
Author contributions
G.Z., J.H. and T.X. performed the experiments and analysed the data. Y.L. and Q.Z. designed and directed the project and wrote the manuscript. G.Z. and Y.L. contributed equally to this work. All the authors discussed the results and commented on the manuscript.
Additional information
Accession codes: The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 979497 (2b), 979496 (9h) and 1031824 (7h). These data can be obtained free of charge from The cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
How to cite this article: Zheng, G. et al. Radical cascade reaction of alkynes with N-fluoroarylsulfonimides and alcohols. Nat. Commun. 6:7011 doi: 10.1038/ncomms8011 (2015).
Supplementary Material
Supplementary Figures 1-126, Supplementary Tables 1-2, Supplementary Methods and Supplementary References
Acknowledgments
We acknowledge support for this work from the National NSF of China (21172033, 21372041 and 21302017).
References
- Hili R. & Yudin A. K. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2, 284–287 (2006). [DOI] [PubMed] [Google Scholar]
- Henkel T., Brunne R. M., Müller H. & Reichel F. Statistical investigation into the structural complementarity of natural products and synthetic compounds. Angew. Chem. Int. Ed. 38, 643–647 (1999). [DOI] [PubMed] [Google Scholar]
- Severin R. & Doye S. The catalytic hydroamination of alkynes. Chem. Soc. Rev. 36, 1407–1420 (2007). [DOI] [PubMed] [Google Scholar]
- Pohlki F. & Doye S. The catalytic hydroamination of alkynes. Chem. Soc. Rev. 32, 104–114 (2003). [DOI] [PubMed] [Google Scholar]
- Alonso F., Beletskaya I. P. & Yus M. Transition-metal-catalyzed addition of heteroatom-hydrogen bonds to alkynes. Chem. Rev. 104, 3079–3160 (2004). [DOI] [PubMed] [Google Scholar]
- Müller T. E. & Beller M. Metal-initiated amination of alkenes and alkynes. Chem. Rev. 98, 675–703 (1998). [DOI] [PubMed] [Google Scholar]
- Li J. & Neuville L. Copper-catalyzed oxidative diamination of terminal alkynes by amidines: synthesis of 1,2,4- trisubstituted imidazoles. Org. Lett. 15, 1752–1755 (2013). [DOI] [PubMed] [Google Scholar]
- Zeng J., Tan Y. J., Leow M. L. & Liu X.-W. Copper(II)/iron(III) Co-catalyzed intermolecular diamination of alkynes: facile synthesis of imidazopyridines. Org. Lett. 14, 4386–4389 (2012). [DOI] [PubMed] [Google Scholar]
- He W., Li C. & Zhang L. An efficient [2+2+1] synthesis of 2,5-disubstituted oxazoles via gold-catalyzed intermolecular alkyne oxidation. J. Am. Chem. Soc. 133, 8482–8485 (2011). [DOI] [PubMed] [Google Scholar]
- Hirano K., Satoh T. & Miura M. Copper-catalyzed annulative amination of ortho-alkynylphenols with hydroxylamines: synthesis of 3-aminobenzofurans by umpolung amination strategy. Org. Lett. 13, 2395–2397 (2011). [DOI] [PubMed] [Google Scholar]
- Luo Y., Ji K., Li Y. & Zhang L. Tempering the reactivities of postulated α-Oxo gold carbenes using bidentate ligands: implication of tricoordinated gold intermediates and the development of an expedient bimolecular assembly of 2,4-disubstituted oxazoles. J. Am. Chem. Soc. 134, 17412–17415 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A. M. et al. Gold-catalyzed 1,2-difunctionalizations of aminoalkynes using only N- and O- containing oxidants. J. Am. Chem. Soc. 133, 15372–15375 (2011). [DOI] [PubMed] [Google Scholar]
- Karur S. et al. A catalytic reaction of alkynes via multiple-site functionalization. J. Am. Chem. Soc. 125, 13340–13341 (2003). [DOI] [PubMed] [Google Scholar]
- Li M., Yuan H., Zhao B., Liang F. & Zhang J. Alkyne aminohalogenation enabled by DBU-activated N-haloimides: direct synthesis of halogenated enamines. Chem. Commun. 50, 2360–2363 (2014). [DOI] [PubMed] [Google Scholar]
- Yu D., Sum Y. N., Ean A. C. C., Chin M. P. & Zhang Y. Acetylide ion (C22-) as a synthon to link electrophiles and nucleophiles: a simple method for enaminone synthesis. Angew. Chem. Int. Ed. 52, 5125–5128 (2013). [DOI] [PubMed] [Google Scholar]
- Wille U. Radical cascades initiated by intermolecular radical addition to alkynes and related triple bond systems. Chem. Rev. 113, 813–853 (2013). [DOI] [PubMed] [Google Scholar]
- Wille U., Heuger G. & Jargstorff C. N-Centered radicals in self-terminating radical cyclizations: experimental and computational studies. J. Org. Chem. 73, 1413–1421 (2008). [DOI] [PubMed] [Google Scholar]
- Wille U., Krüger O., Kirsch A. & Lüning U. Imidyl radicals, radical addition of N-bromophthalimide to linear and cyclic alkynes. Eur. J. Org. Chem. 1999, 3185–3189 (1999). [Google Scholar]
- Zhang H. et al. Copper-catalyzed intermolecular aminocyanation and diamination of alkenes. Angew. Chem. Int. Ed. 52, 2529–2533 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang H., Song Y., Zhao J., Zhang J. & Zhang Q. Regioselective radical aminofluorination of styrenes. Angew. Chem. Int. Ed. 53, 11079–11083 (2014). [DOI] [PubMed] [Google Scholar]
- Xiong T., Li Y., Mao L., Zhang Q. & Zhang Q. Palladium-catalyzed allylic C−H amination of alkenes with N-fluorodibenzenesulfonimide: water plays an important role. Chem. Commun. 48, 2246–2248 (2012). [DOI] [PubMed] [Google Scholar]
- Ni Z. et al. Highly regioselective copper-catalyzed benzylic C−H amination by N-fluorobenzenesulfonimide. Angew. Chem. Int. Ed. 51, 1244–1247 (2012). [DOI] [PubMed] [Google Scholar]
- Sun K., Li Y., Xiong T., Zhang J. & Zhang Q. Palladium-catalyzed C-H aminations of anilides with N-fluorobenzenesulfonimide. J. Am. Chem. Soc. 133, 1694–1697 (2011). [DOI] [PubMed] [Google Scholar]
- Xiong T., Li Y., Lv Y. & Zhang Q. Remote amide-directed palladium-catalyzed benzylic C–H amination with N-fluorobenzenesulfonimide. Chem. Commun. 46, 6831–6833 (2010). [DOI] [PubMed] [Google Scholar]
- Stratmann K. et al. Welwitindolinones, Unusual alkaloids from the blue-green algae hapalosiphon welwitschii and westiella intricata. relationship to fischerindoles and hapalindoles. J. Am. Chem. Soc. 116, 9935–9942 (1994). [Google Scholar]
- Hijazi Y. & Boulieu R. Contribution of CYP3A4, CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demetaylation of ketamine in human liver microsomes. Drug Metab. Dispos. 30, 853–858 (2002). [DOI] [PubMed] [Google Scholar]
- Varenhorst C. et al. Genetic variation of CYP2C19 affects both pharmacokinetic and pharmacodynamic responses to clopidogrel but not prasugrel in aspirin-treated patients with coronary artery disease. Eur. Heart J. 30, 1744–1752 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell T. N. & Allen W. E. A regiospecific synthesis of 1,4-disubstituted imidazoles. J. Org. Chem. 59, 1589–1590 (1994). [Google Scholar]
- Langer P. & Bodtke A. Sequential cyclizations of 2-isothiocyanatobenzonitrile and 2-isocyanatobenzonitrile with α-aminoketones. Tetrahedron Lett. 44, 5965–5967 (2003). [Google Scholar]
- Frantz D. E. et al. Synthesis of substituted imidazoles via organocatalysis. Org. Lett. 6, 843–846 (2004). [DOI] [PubMed] [Google Scholar]
- Bunescu A., Piemontesi C., Wang Q. & Zhu J. Heteroannulation of arynes with N-aryl α-aminoketones for the synthesis of unsymmetrical N-aryl-2,3-disubstituted indoles: an aryne twist of Bischler–Möhlau indole synthesis. Chem. Commun. 49, 10284–10286 (2013). [DOI] [PubMed] [Google Scholar]
- Ager D. J., Prakash I. D. & Schaad R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 96, 835–876 (1996). [DOI] [PubMed] [Google Scholar]
- Reetz M. T. New approaches to the use of amino acids as chiral building blocks in organic synthesis [new synthetic methods (85)]. Angew. Chem. Int. Ed. 30, 1531–1546 (1991). [Google Scholar]
- Li G.-Q., Dai L.-X. & You S.-L. Thiazolium-derived N-heterocyclic carbene-catalyzed cross-coupling of aldehydes with unactivated imines. Chem. Commun. 8, 852–854 (2007). [DOI] [PubMed] [Google Scholar]
- Murry J. A. et al. Synthesis of α-amido ketones via organic catalysis: thiazolium-catalyzed cross-coupling of aldehydes with acylimines. J. Am. Chem. Soc. 123, 9696–9697 (2001). [DOI] [PubMed] [Google Scholar]
- Enders D., Henseler A. & Lowins S. N-Heterocyclic carbene catalyzed nucleophilic acylation of trifluoromethyl ketimines. Synthesis 24, 4125–4128 (2009). [Google Scholar]
- Mennen S. M., Gipson J. D., Kim Y. R. & Miller S. J. Thiazolylalanine-derived catalysts for enantioselective intermolecular aldehyde-imine cross-couplings. J. Am. Chem. Soc. 127, 1654–1655 (2005). [DOI] [PubMed] [Google Scholar]
- DiRocco D. A. & Rovis T. Catalytic asymmetric cross-aza-benzoin reactions of aliphatic aldehydes with N-Boc-protected imines. Angew. Chem. Int. Ed. 51, 5904–5906 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurer M., Frey G., Luu H.-T., Kratzert D. & Streuff J. The cross-selective titanium(III)-catalysed acyloin reaction. Chem. Commun. 50, 5370–5372 (2014). [DOI] [PubMed] [Google Scholar]
- Smith A. M. R. & Hii K. K. Transition metal catalyzed enantioselective α-heterofunctionalization of carbonyl compounds. Chem. Rev. 111, 1637–1656 (2011). [DOI] [PubMed] [Google Scholar]
- Selig P. The electrophilic α-amination of α-alkyl-β-ketoesters with in situ generated nitrosoformates. Angew. Chem. Int. Ed. 52, 7080–7082 (2013). [DOI] [PubMed] [Google Scholar]
- Lv Y. et al. nBu4NI-catalyzed oxidative imidation of ketones with imides: synthesis of α-amino ketones. Chem. Commun. 50, 2367–2369 (2014). [DOI] [PubMed] [Google Scholar]
- Evans R. W., Zbieg J. R., Zhu S., Li W. & MacMillan D. W. C. Simple catalytic mechanism for the direct coupling of α-carbonyls with functionalized amines: a one-step synthesis of plavix. J. Am. Chem. Soc. 135, 16074–16077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C. C. C. & Colacot T. J. Metal-catalyzed α-arylation of carbonyl and related molecules: novel trends in C−C bond formation by C−H bond functionalization. Angew. Chem. Int. Ed. 49, 676–707 (2010). [DOI] [PubMed] [Google Scholar]
- Bellina F. & Rossi R. Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized C-H bonds and some of their synthetic equivalents with aryl halides and pseudohalides. Chem. Rev. 110, 1082–1146 (2010). [DOI] [PubMed] [Google Scholar]
- Danoun G., Tlili A., Monnier F. & Taillefer M. Direct copper-catalyzed α-arylation of benzyl phenyl ketones with aryl iodides: route towards tamoxifen. Angew. Chem. Int. Ed. 51, 12815–12819 (2012). [DOI] [PubMed] [Google Scholar]
- Ge S. & Hartwig J. F. Nickel-catalyzed asymmetric α-arylation and heteroarylation of ketones with chloroarenes: effect of halide on selectivity, oxidation state, and room-temperature reactions. J. Am. Chem. Soc. 133, 16330–16333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesp K. D., Lundgren R. J. & Stradiotto M. Palladium-catalyzed mono-α-arylation of acetone with aryl halides and tosylates. J. Am. Chem. Soc. 133, 5194–5197 (2011). [DOI] [PubMed] [Google Scholar]
- Kaneko K., Yoshino T., Matsunaga S. & Kanai M. Sultam synthesis via Cu-catalyzed intermolecular carboamination of alkenes with N-Fluorobenzenesulfonimide. Org. Lett. 15, 2502–2505 (2013). [DOI] [PubMed] [Google Scholar]
- Boursalian G. B., Ngai M. Y., Hojczyk K. N. & Ritter T. Pd-catalyzed aryl C−H imidation with arene as the limiting reagent. J. Am. Chem. Soc. 135, 13278–13281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. & Studer A. Copper-catalyzed intermolecular aminoazidation of alkenes. Org. Lett. 16, 1790–1793 (2014). [DOI] [PubMed] [Google Scholar]
- Du Y. et al. Asymmetric reductive Mannich reaction to ketimines catalyzed by a Cu(I) complex. J. Am. Chem. Soc. 130, 16146–16147 (2008). [DOI] [PubMed] [Google Scholar]
- Shintani R., Takeda M., Tsuji T. & Hayashi T. Rhodium-catalyzed asymmetric arylation of N-tosyl ketimines. J. Am. Chem. Soc. 132, 13168–13169 (2010). [DOI] [PubMed] [Google Scholar]
- Shintani R., Takeda M., Soh Y.-T., Ito T. & Hayashi T. Rhodium-catalyzed asymmetric addition of potassium organotrifluoroborates to N-sulfonyl Ketimines. Org. Lett. 13, 2977–2979 (2011). [DOI] [PubMed] [Google Scholar]
- Nishimura T., Noishiki A., Tsui G. C. & Hayashi T. Asymmetric synthesis of (triaryl)methylamines by rhodium catalyzed addition of arylboroxines to cyclic N-sulfonyl ketimines. J. Am. Chem. Soc. 134, 5056–5059 (2012). [DOI] [PubMed] [Google Scholar]
- Clayden J., Donnard M., Lefranc J. & Tetlow D. J. Quaternary centres bearing nitrogen (α-tertiary amines) as products of molecular rearrangements. Chem. Commun. 47, 4624–4639 (2011). [DOI] [PubMed] [Google Scholar]
- Miura T., Tanaka T., Biyajima T., Yada A. & Murakami M. One-pot procedure for the introduction of three different bonds onto terminal alkynes through N-sulfonyl-1,2,3-triazole intermediates. Angew. Chem. Int. Ed. 52, 3883–3886 (2013). [DOI] [PubMed] [Google Scholar]
- Gheorghe A., Quiclet-Sire B., Vilaa X. & Zard S. Synthesis of 3-arylpiperidines by a radical 1,4-aryl migration. Org. Lett. 7, 1653–1656 (2005). [DOI] [PubMed] [Google Scholar]
- Kong W., Casimiro M., Merino E. & Nevado C. Copper-catalyzed one-pot trifluoromethylation/aryl migration/desulfonylation and C(sp2)−N bond formation of conjugated tosyl amides. J. Am. Chem. Soc. 135, 14480–14483 (2013). [DOI] [PubMed] [Google Scholar]
- Kong W., Merino E. & Nevado C. Arylphosphonylation and arylazidation of activated alkenes. Angew. Chem. Int. Ed. 53, 5078–5082 (2014). [DOI] [PubMed] [Google Scholar]
- Gao P. et al. Copper-catalyzed one-pot trifluoromethylation/aryl migration/carbonyl formation with homopropargylic alcohols. Angew. Chem. Int. Ed. 53, 7629–7633 (2014). [DOI] [PubMed] [Google Scholar]
- Song Z.-L., Fan C.-A. & Tu Y.-Q. Semipinacol rearrangement in natural product synthesis. Chem. Rev. 111, 7523–7556 (2011). [DOI] [PubMed] [Google Scholar]
- Paquette L. A. & Hofferberth J. E. Org. React 62, 477–567 (2003). [Google Scholar]
- Zhang X., Staples R. J., Rheingold A. L. & Wulff W. D. Catalytic asymmetric α-iminol rearrangement: new chiral platform. J. Am. Chem. Soc. 136, 13971–13974 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-126, Supplementary Tables 1-2, Supplementary Methods and Supplementary References