Abstract
Background
Besides their health benefits, dietary omega-3 fatty acids (n-3 PUFAs) can impair host resistance to intracellular pathogens. Previously, we and others have showed that n-3 PUFA–treated macrophages poorly control Mycobacterium tuberculosis infection in vitro.
Methods
Wild-type and fat-1 transgenic mice were infected with virulent H37Rv M. tuberculosis via the aerosol route. We evaluated bacteriological and histopathological changes in lungs, as well as differences in activation and antimycobacterial capacity in primary macrophages ex vivo.
Results
fat-1 mice were more susceptible to tuberculosis, as demonstrated by higher bacterial loads and less robust inflammatory responses in lungs. Macrophages obtained from fat-1 mice were more readily infected with M. tuberculosis in vitro, compared with wild-type macrophages. This impaired bacterial control in cells from fat-1 mice correlated with reduced proinflammatory cytokine secretion, impaired oxidative metabolism, and diminished M. tuberculosis–lysotracker colocalization within phagosomes.
Conclusions
We showed that endogenous production of n-3 PUFAs in fat-1 mice increases their susceptibility to tuberculosis, which could be explained in part by diminished activation and antimycobacterial responses in cells from fat-1 mice. These data suggest that n-3 PUFA–supplemented diets might have a detrimental effect on immunity to M. tuberculosis and raise concerns regarding the safety of omega-3 dietary supplementation in humans.
Tuberculosis continues to be a public health problem worldwide, with more than 1 billion people infected. An effective cellular immune response is required for resistance to tuberculosis and is characterized by the recruitment and activation of T cells and macrophages, which can be modulated by nutritional components. Protein malnutrition [1], hypercholesterolemia [2], and some dietary lipids [3] are risk factors for tuberculosis progression. It has been suggested that dietary omega-3 fatty acids (n-3 PUFAs) impair the immune response against Mycobacterium tuberculosis [3–5], but the mechanisms involved have not been fully elucidated.
Dietary n-3 PUFAs have anti-inflammatory properties. These lipids down-regulate immune cell activation and functionality by altering lipid membrane composition, gene expression, and generation of cytokines and other mediators [6]. Such anti-inflammatory effects make n-3 PUFAs useful in prevention and treatment of cardiovascular diseases [7] and cancer [8]. The interest in their beneficial properties arose from epidemiological studies of the Inuit people in Greenland, who have a low incidence of coronary heart disease that is associated with increased n-3 PUFA in-take [9]. However, this population also has an increased incidence of tuberculosis [10]. Indeed, we and others demonstrated that n-3 PUFA–fed guinea pigs infected with M. tuberculosis have poor bacterial control and more-severe disease [4, 11, 12]. However, the cellular mechanisms responsible for the detrimental n-3 PUFA effect are unknown. n-3 PUFAs interfere with immune cell activation. Diets enriched in n-3 PUFAs suppressed several functions of T cells [13–15] and macrophages [16]. We showed that incorporation of the n-3 PUFA docosahexaenoic acid reduced the ability of J774A.1 murine macrophages to kill M. tuberculosis by impairing cell activation, proinflammatory cytokine production, reactive oxygen intermediate generation, and phagosome maturation (D.L.B., L. Ly, Y.-Y.F., R.S.C., and D.N.M., unpublished data). Anes et al [3] and Jordao et al [5] reported that n-3 PUFAs favor mycobacterial survival by impairing phagosomal actin recruitment. Therefore, we hypothesized that n-3 PUFAs would impair host resistance to pulmonary M. tuberculosis infection in vivo.
Because mammals lack the desaturase enzyme needed for the synthesis of n-3 PUFAs, these fatty acids must be obtained from the diet. Most of the n-3 PUFA studies in vivo use dietary supplementation; however, many variables arise from this approach because of differences in diet composition, diet preparation, and food intake. Kang et al [17] developed a fat-1 transgenic mouse model that endogenously produces n-3 PUFAs. fat-1 mice express the fat-1 gene from Caenorhabditis elegans, which encodes an n-3 desaturase that catalyzes n-3 PUFA synthesis from n-6 PUFA substrates. fat-1 mice have been used to elucidate the protective effect of n-3 PUFAs in inflammatory diseases such as colitis [18], melanoma [19], and others [20– 24]. We have chosen fat-1 mice as a relevant alternative to diet modulation to evaluate the role of n-3 PUFAs during tuberculosis infection.
Here, we demonstrate for the first time, to our knowledge, that the endogenous enrichment of n-3 PUFAs in fat-1 mice increased their susceptibility to pulmonary tuberculosis infection, in part by impairing macrophage activation and anti-mycobacterial capacity.
MATERIALS AND METHODS
Animals
Heterozygous transgenic fat-1 mice on a C57BL/6 background were generated as described elsewhere [21]. Nontransgenic littermate wild-type mice were used as controls. Animals were housed 5 per cage in a controlled temperature and humidity environment with a 12-h light-dark cycle and under pathogen-free conditions. Male mice, 8–10 weeks of age, were used in all experiments. Wild-type and fat-1 mice were fed the same special AIN-76A rodent diet with 10% safflower oil (Research Diets #D03092902) [18, 21]. Mice had access to food and water ad libitum, and all procedures were approved by the Institutional Animal Care and Use committee at Texas A&M University. Genotype and phenotype were confirmed by real-time polymerase chain reaction and gas chromatography, respectively, according to protocols published elsewhere [21]. M. tuberculosis–infected animals were restricted to a biosafety level 3 containment facility.
Aerosol infection and necropsy
Virulent M. tuberculosis H37Rv (ATCC 27294) was cultured in Middlebrook 7H9 medium (Becton Dickinson), and stocks were prepared and stored at −80° C before use [25]. For infection, stocks were thawed, sonicated, and passed through a 28G needle 15 times [26]. Mice were infected via the pulmonary route in an aerosol chamber (University of Wisconsin Engineering Shops), according the protocol published elsewhere [27, 28]. Confirmation of the initial infection level was determined at 1 day postinfection. Five animals per group per time point were euthanized by intramuscular injection of sodium pentobarbital (100 mg/mL Sleepaway; Fort Dodge Laboratories).
Bacterial load determination
The right lower lung lobe and a portion of the spleen were removed aseptically, homogenized separately, diluted, and plated onto Middlebrook 7H10 agar (Becton Dickinson), as described elsewhere [29]. After 3– 4 weeks of incubation at 37°C, the number of colonies were counted and expressed as mean log10 colony forming units (CFUs) per tissue.
Histological evaluation
Histological sections of formalinfixed lung and spleen were stained with hematoxylin and eosin and examined by a Board-Certified pathologist in a blinded fashion. The percentage of tissue involved in the inflammatory response was determined by morphometric analysis with use of the Image J 11.4 software. The evaluation of lung sections was performed using scores of 0–3 for the following criteria: (1) the degree of inflammation (0, no inflammation, occasional inflammatory cells; 1, mild, increased number of inflammatory cells; 2, moderate, confluent inflammatory cells; 3, severe, extended infiltrate; and (2) granulomatous organization (0, tissues with no lesions; 1, infiltrates exhibiting the most organization [granuloma-like lesions, central area of macrophages surrounded by lymphocytes]; 2, cell aggregates with slight peripheralization of lymphocytes; 3, least organized granulomatous foci [with scatterings of inflammatory cells loosely grouped]) [18, 21, 29].
Isolation and infection of elicited peritoneal cells
For ex vivo experiments, peritoneal cells were collected according to our protocol published elsewhere [28]. Contaminating erythrocytes were removed with ACK lysing buffer, and cell counts and viability were determined by trypan blue exclusion. Cells were plated and allowed to adhere. After 2 h, nonadherent cells were removed, and adherent cells were incubated in media containing 2% fetal bovine serum (Atlanta Biol). Virulent H37Rv M. tuberculosis (ATCC 27294) was used as described elsewhere [25]. Adherent cells were infected at a multiplicity of infection of 10 or 20, according to the type of experiment. Cell monolayers were washed with phosphate-buffered saline (Gibco) and incubated with 50-μg/mL gentamicin (Gibco) to kill extracellular bacteria [30]. Cells were spun and stained with Diff Quik (Dade Behring) for differential leukocyte count.
Fatty acid analysis
Total lipids were extracted by the method of Folch [31]. Total phospholipids were separated using chloroform, methanol, acetic acid, and water as solvents and were transesterified in presence of 6% methanolic HCl. Fatty acids methyl esters were analyzed by capillary gas chromatography as described elsewhere [32, 33]. Peaks of resolved fatty acids were identified by comparing the relative retention times with those of a known standard (GLC 463; Nu Chek Prep). Results are expressed as percentage of total fatty acids and as a ratio of n-6 (20:4n-6 + 22:4n-6 + 22:5n-6) to n-3 (20:5n-3 + 22:5n-3 + 22:6n-3).
Intracellular bacterial trafficking and survival
Cell mono-layers were infected with a green fluorescent protein (GFP)– expressing virulent strain (H37Rv) of M. tuberculosis, kindly provided by Dr Scott Franzblau (University of Chicago) [34]. Nuclei were counterstained with 1 mmol/L Hoescht 33342 (Fluka). Extracellular bacteria were labeled using a polyclonal antibody E193.1.11.WCL against whole cell lysate of M. tuberculosis, kindly provided by Dr Karen Dobos-Elder (Colorado State University). Serial sections across entire cells were taken to confirm the intracellular localization with use of a DSU1X81 Olympus microscope. The percentage of infected cells was determined by counting at least 100 cells per slide. For the CFU assay, cell lysates were plated on 7H10 Middlebrook agar (Becton Dickinson). The CFU counts were determined after 3–4 weeks of incubation at 37°C in 5% CO2, as described elsewhere [29].
Cytokine production
Culture supernatants were harvested and assessed for tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemotactic protein–1 and IL-1β by enzyme-linked immunosorbent assay (EBioscience), according to the manufacturer's protocol.
Phagolysosome maturation
Cells seeded onto #1 thickness glass coverslips were infected with GFP-expressing bacteria (multiplicity of infection, 20) and incubated with 50 nmol/L Lysotracker Red DND99 (Invitrogen) for 2 h before and during the infection. Cells were gentamycin-treated, formaldehydefixed, and mounted on glass slides with Slowfade. Nuclei were counterstained with 1 mmol/L Hoescht 33342 (Fluka). The percentage of lysotracker-positive GFP-fluorescing mycobacterial phagosomes was determined by counting at least 100 phagosomes per slide.
Reactive oxygen and nitrogen species generation
Intra-cellular reactive oxygen levels were measured by staining with 5 mmol/L dihydroethidium (Invitrogen), according to Carter et al [35]. Stained cells were fixed in 2% paraformaldehyde in phosphate-buffered saline and were analyzed using a FACSCalibur flow cytometer (Becton Dickinson). Nitric oxide (NO) levels in culture supernatants were measured using the Griess Reagent (Promega), according to the manufacturer's protocol.
Statistical analysis
Data analysis was performed with GraphPad software (GraphPad) by using the Student t test for samples with equal variances, considering P values <.05 to be significant. Values are presented as means and error bars represent standard errors (SEM). Significant correlations were determined by calculating the Pearson coefficient.
RESULTS
Nutritional status and weight gain
There were no significant differences in body weight, food intake or water consumption between fat-1 and wild-type mice, throughout the feeding period or the course of the study. The mean starting weight (± SEM) was 30.9 ± 1.19 g for wild-type mice and 31.4 ± 2.02 g for fat-1 mice, and the mean final weights were 31.3 ± 0.41 g and 30.3 ± 0.89 g, respectively (P > .05).
Mouse fatty acid profiles
Both fat-1 and wild-type mice were fed the same diet enriched in n-6 PUFAs. Analysis of total lipids from tail snips and peritoneal macrophages showed distinct fatty acid profiles, characterized by n-3 PUFA enrichment only in transgenic mice (Table 1). In tail snips, the mean n-6/ n-3 PUFA ratio (± SEM) was 1.81 ± 0.15 in fat-1 and 28.24 ± 0.91 in wild-type mice (P < .001). The mean concentration of docosahexaenoic acid (± SEM) in fat-1 mice was 3.29% ± 0.26%, compared with 0.01% ± 0.01% in wild-type mice (P < .001). Conversely, there were significantly lower levels of n-6 fatty acids in fat-1 mice. For example, the mean percentage (± SEM) of 22:4n-6 in cells from fat-1 mice was 0.32% ± 0.29%, compared with 2.46% ± 0.17% in cells from wild-type mice (P < .01). Similarly, in the thioglycollate-elicited macrophages used in ex vivo experiments, the ratio of n-6 to n-3 was significantly lower in fat-1 cells (P < .001).
Table 1.
Lipid Profiles in Tail and Peritoneal Macrophages from Wild-Type (WT) and fat-1 Mice
| Mean mol per 100 mol total fatty acids ± SEM |
||||
|---|---|---|---|---|
| Tail Snip |
Peritoneal Macrophages |
|||
| Fatty acid | WT | fat-1 | WT | fat-1 |
| 18:2n-6 | 17.43 ± 0.8 | 15.87 ± 0.9 | 7.4 ± 0.4 | 10.26 ± 0.4 |
| 18:3n-3 | 0.05 ± 0.1 | 1.70 ± 0.1a | 1.94 ± 0.1 | 2.12 ± 0.2 |
| 20:4n-6 | 5.16 ± 0.3 | 0.57 ± 0.2a | 17.66 ± 1.2 | 14.31 ± 1.2 |
| 20:5n-3 | 0.05 ± 0.1 | 1.93 ± 0.2a | 0 ± 0 | 1.68 ± 0.6a |
| 22:4n-6 | 2.46 ± 0.2 | 0.32 ± 0.3a | 4.27 ± 0.2 | 0.99 ± 0.2a |
| 22:5n-6 | 3.32 ± 0.2 | 0.06 ± 0.1a | 3.00 ± 0.2 | 0.58 ± 0.3a |
| 22:5n-3 | 0.05 ± 0.02 | 3.04 ± 0.21a | 0 ± 0 | 1.89 ± 0.47a |
| 22:6n-3 | 0.01 ± 0.01 | 3.29 ± 0.26a | 0 ± 0 | 1.52 ± 0.17a |
| n:6/n-3 ratio | 28.24 ± 0.91 | 1.81 ± 0.15a | 24.93 ± 1.65 | 4.25 ± 2.05 |
NOTE. Total lipids were extracted and analyzed by gas chromatography, as described in the Materials and Methods. Only selected major fatty acids (>1 mol %) are reported. SEM, standard error of the mean.
P<.05.
fat-1 mice are more susceptible to pulmonary M. tuberculosis infection
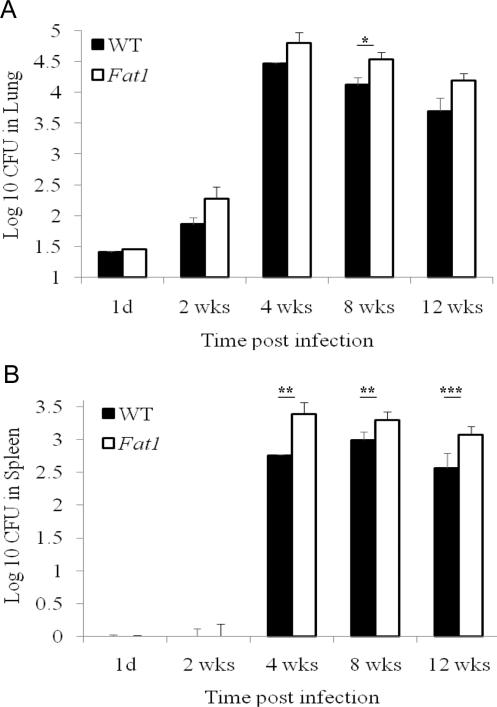
To evaluate the effect of n-3 PUFA enrichment on the resistance to tuberculosis, we determined bacterial loads in lungs and spleens from aerosol-infected animals as a measure of host resistance to the infection. The mean number (± SEM) of implanted bacteria in lung was not significantly different between fat-1 (1.41 ± 0.02 log10CFU) and wild-type (1.45 ± 0.02 log10CFU) mice at day 1 postinfection (P > .05). Figure 1A shows that M. tuberculosis grew progressively until 4 weeks, after which the infection was controlled and held at a stationary level of ~4.5 log10CFU in lungs and 3 log10CFU in spleens (Figure 1B). M. tuberculosis was found in spleens only after extrapulmonary dissemination occurred about 2 weeks postinfection. We observed significantly increased mean bacterial counts (± SEM) in tissues from fat-1 mice (4.53 ± 0.12 log10CFU), compared with those from wild-type animals (4.12 ± 0.12 log10CFU) at 8 weeks after challenge (P < .005) (Figure 1A). In spleens, the n-3 PUFA enrichment was also associated with greater bacterial loads at 4 (P < .05), 8 (P < .05), and 12 weeks (P < .01) (Figure 1B).
Figure 1.
Increased bacterial loads in fat-1 mice. Animals were infected with Mycobacterium tuberculosis via aerosol infection. At 1 day and at 2, 4, 8, and 12 weeks, mice were euthanized, and the numbers of viable bacteria in lung and spleen were determined. Bacterial counts were estimated by the colony forming unit (CFU) assay, as described in the Materials and Methods. Data show bacterial survival as log10 CFU (mean ± standard error of the mean) in fat-1 (dashed line) versus wild-type (WT) mice (solid line). *P < .005; **P < .05; ***P < .01.
fat-1 mice exhibited reduced pulmonary inflammation
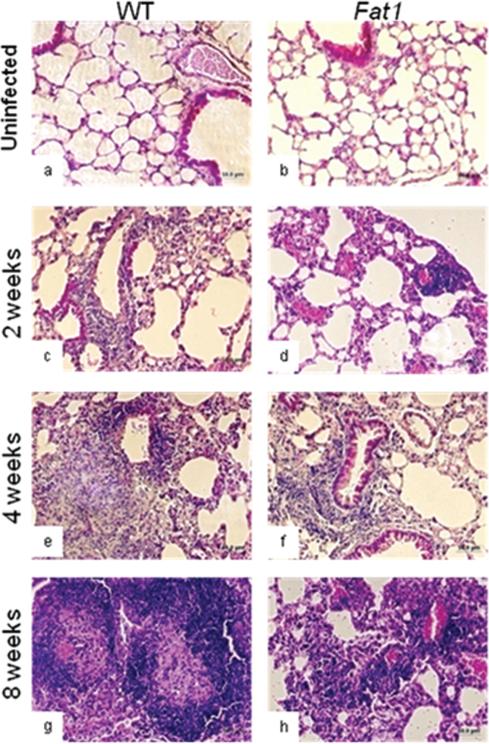
To evaluate the effect of n-3 PUFA enrichment on the cellular inflammatory response to tuberculosis in lungs, we compared the extent and organization of the inflammatory infiltrate in fat-1 and wild-type animals in hematoxylin and eosin–stained sections. Inflammatory changes were not observed in uninfected lungs from fat-1 and wild-type animals (Figure 2A and 2B). Two weeks following aerosol infection, histopathological changes in the lungs of both wild-type and fat-1 mice included scattered minimal accumulations of lymphocytes within the pulmonary parenchyma, as well as perivascular recruitment of lymphocytes (Figure 2C and 2D). At 4 weeks, more focal dense accumulations of lymphocytes and macrophages were distributed randomly within the sections. At this interval, early granulomatous lesions were seen in wild-type mice, in contrast to less pronounced inflammatory responses in fat-1 mice (Figure 2E and 2F). By 8 weeks, massive inflammation was found in the lungs of wild-type mice accompanied by perivasculatis, peribroncholitis, and thickening of parenchymal walls, with few regions organized into discrete foci of macrophages surrounded by a zone of lymphocytes (granulomas-like lesions) (Figure 2G). Conversely, fat-1 mice exhibited diffused poorly-formed and less–circumscribed granulomatous accumulations with lack of granuloma-like structures (Figure 2G and 2H). The granulomatous reactions in lungs of infected wild-type mice appeared to be more cellular and better organized than those from fat-1 animals (Figure 2E and 2G). We determined the percentage of lung tissue compromised by the inflammatory response and quantified the degree of inflammation and the organization of the granulomatous response. The analysis of the percentage of involved lung section area and the degree of inflammation confirmed that infected fat-1 mice had a reduced inflammatory response after M. tuberculosis infection (Table 2). On the other hand, when the organization of granulomatous foci was scored, the nature of the lesion differed between fat-1 and wild-type animals. fat-1 sections were scored lower because of the diffuse nature of cell aggregate infiltration, compared with mature granuloma-like lymphoid aggregates in wild-type lungs (Table 2). A toxic effect of n-3 PUFAs was ruled out by histopatho-logical analysis of uninfected wild-type and fat-1 tissues.
Figure 2.
Representative hematoxylin and eosin–stained lung sections from fat-1 (panels b, d, f, and h) and wild-type (WT; panels a, c, e, and g) mice after infection with Mycobacterium tuberculosis. The total magnification is ×200. Animals were infected with M. tuberculosis via aerosol infection. At 1 day and at 2, 4, and 8 weeks mice were euthanized, and histological changes in lung and spleen were evaluated as described in the Materials and Methods.
Table 2.
Semiquantitative Analysis of the Extent and Organization of the Inflammatory Response in Hematoxylin and Eosinstained Lung Sections
| Variable | WT | fat1 |
|---|---|---|
| Percentage of area compromiseda | ||
| Time postinfection | ||
| Uninfected | 20.98 ± 1.8 | 22.46 ± 0.8b |
| 1 day | 29.28 ± 1.1 | 26.49 ± 2.7b |
| 2 weeks | 26.19 ± 1.1 | 22.68 ± 1.2b |
| 4 weeks | 32.10 ± 1.3 | 29.05 ± 1.0b |
| 8 weeks | 44.67 ± 1.2 | 38.91 ± 1.00c |
| Degree of inflammationd | ||
| Time postinfection | ||
| Uninfected | 0.4 ± 0.1 | 0.30 ± 0.1 |
| 1 day | 1.0 ± 0.2 | 0.5 ± 0.2b |
| 2 weeks | 1.4 ± 0.1 | 0.7 ± 0.1c |
| 4 weeks | 1.9 ± 0.2 | 1.4 ± 0.2b |
| 8 weeks | 2.5 ± 0.1 | 1.7 ± 0.2c |
| Granulomatous organizatione | ||
| Time postinfection | ||
| Uninfected | 0 ± 0 | 0± 0 |
| 1day | 0± 0 | 0± 0 |
| 2 weeks | 0 ± 0 | 0± 0 |
| 4 weeks | 1.8 ± 0.1 | 2.5 ± 0.2b |
| 8 weeks | 1.8 ± 0.1 | 2.7 ± 0.1b |
The percentage of tissue involved in the inflammatory response was determined as described in Materials and Methods.
P<.01.
P<.001.
The degree of inflammation was scored from 0 to 3 (0, no inflammation; 1, mild; 2, confluent inflammatory cells; 3, extended infiltrate).
The granulomatous organization was scored from 0 to 3 (0, no lesions; 1, well-organized infiltrate; 2, reduced organization; 3, the least organized granulomatous foci).
fat-1-elicited macrophages had reduced ability to control M. tuberculosis infection in vitro
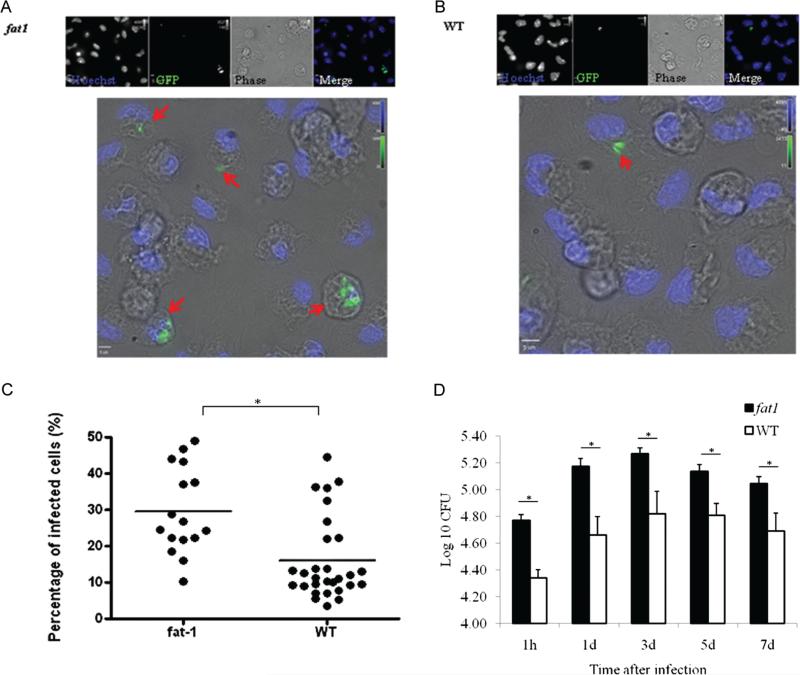
To elucidate the potential mechanisms by which n-3 PUFAs alter the resistance to tuberculosis in fat-1 mice, additional experiments were conducted using primary cells infected ex vivo. Thioglycollate-elicited, adherent peritoneal cells were predominantly macrophages (>90%) as revealed by cell morphology and staining properties (data not shown). Macrophages from fat-1 mice had a reduced ability to eliminate M. tuberculosis infection. Figure 3 shows representative fluorescent images of the higher percentage of infection associated with n-3 PUFA enrichment in cells from fat-1 mice (Figure 3A), compared with wild-type mice (Figure 3B). At 1 h postinfection, cells from fat-1 mice exhibited a significantly higher percentage of infection (mean ± SEM, 44.82% ± 2.24%), compared with wild-type macrophages (mean ± SEM, 29.83% ± 2.69%) (Figure 3C). In Figure 3D, exponential bacterial growth within macrophages was observed between 1 h and 3 days postinfection, after which infection was controlled and held at a stationary level. At 1 h postinfection, cells from fat-1 mice had a significant 1.10-fold increase in bacterial loads, compared with wild-type cells (P < .001), and the n-3 PUFA levels were positively correlated with increased CFU values (R = 0.54; P < .001). Cell viability was not affected throughout the entire experiment (data not shown).
Figure 3.
Increased mycobacterial permisiveness in fat-1 macrophages. Cells were infected with green fluorescent protein (GFP)-expressing Mycobacterium tuberculosis for 1 h, and the relative percentage of M. tuberculosis–infected cells was quantified by fluorescent microscopy, as described in the Materials and Methods. Representative fluorescent images of the increased bacterial accumulation in fat-1 cells (A) at 1 h postinfection, compared with wild-type (WT) cells (B). Arrows show infected cells. Data are representative of 3 independent experiments. C, Quantitative data represent the percentage of infected cells. D, Bacterial counts were estimated at different time points by the colony forming unit (CFU) assay, as described in the Materials and Methods. Data show bacterial survival as log10 CFU (mean ± standard error of the mean; n = 10). *P < .001.
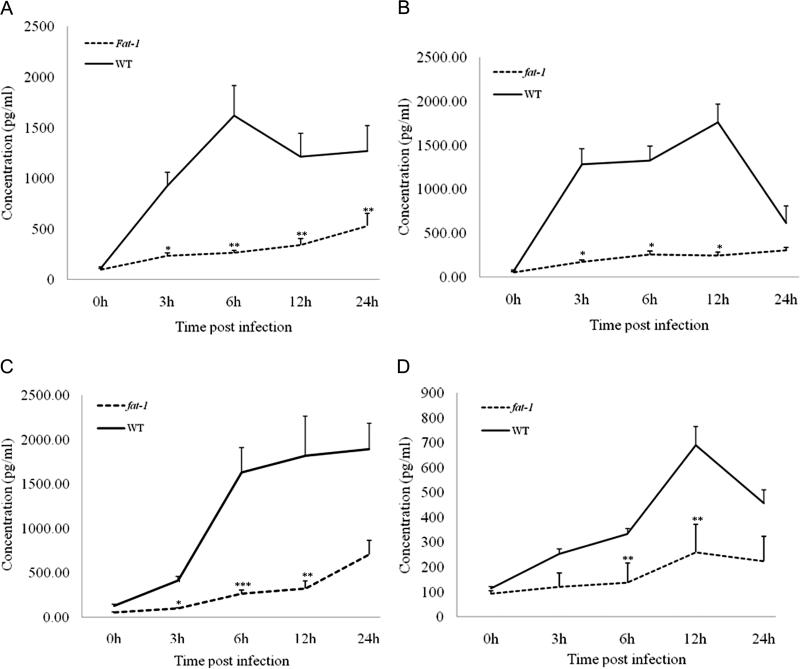
fat-1 elicited macrophages produced reduced levels of pro-inflammatory cytokines after M. tuberculosis infection
n-3 PUFA enrichment significantly reduced TNF-a protein levels (Figure 4A). Cells from fat-1 mice produced only ~15% of the TNF-α protein levels (mean level ± SEM, 264.15 ± 22.16 pg/ mL) observed in macrophages from wild-type animals (mean level ± SEM, 1619.18 ± 298.25 pg/mL) at 6 h postinfection (P < .05). Furthermore, levels of IL-6 (Figure 4B), IL-1β (Figure 4C), and monocyte chemotactic protein–1 (Figure 4D) were also down-regulated in cells from fat-1 mice. We observed a similar suppressive effect in cells from fat-1 mice stimulated with lipopolysaccharide (data not shown).
Figure 4.
Reduced cytokine production in infected fat-1 macrophages. TNF-α (A), interleukin-6 (B), interleukin-1β (C), and monocyte chemotactic protein–1 (D) protein concentrations were quantified in culture supernatants at various times following infection with virulent Mycobacterium tuberculosis H37Rv by enzyme-linked immunosorbent assay, as described in the Materials and Methods. *P < .001; **P < .05; ***P < .01
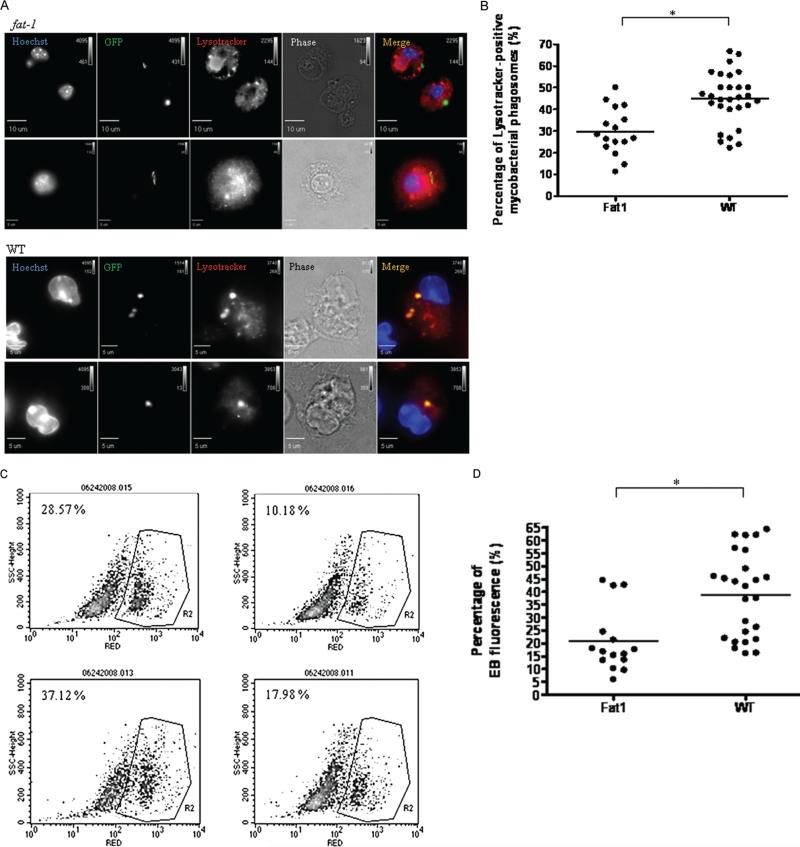
Infected fat-1–elicited macrophages showed impaired co-localization of lysotracker with mycobacterial phagosomes and reduced oxidative response
The acidotropic probe lyso-tracker colocalizes with lysosomal markers in M. tuberculosis– containing phagosomes [36]. Figure 5A shows representative fluorescence images of lysotracker recruitment to mycobacterial phagosomes. At 1 h postinfection (Figure 5B), the percentage of lysotracker-positive M. tuberculosis–containing phagosomes was reduced in cells from fat-1 mice (mean percentage ± SEM, 29.83% ± 2.69% vs 44.82% ± 2.24%; P < .001), suggesting a defective phagolysosome maturation. The colocalization of other late-endosomal markers was not addressed in this study.
Figure 5.
Acquisition of lysotracker by green fluorescent protein (GFP) Mycobacterium tuberculosis–containing phagosomes and reactive oxygen generation was reduced in infected fat-1 macrophages. Phagosomal maturation was defined based on lysotracker colocalization and visualized by fluorescent microscopy, as described in the Materials and Methods. A, Representative images of the reduced colocalization in fat-1 macrophages, compared with wild-type (WT) macrophages. Data are representative of 3 independent experiments. B, Quantitative data show the percentage of lysotracker-positive mycobacterial phagosomes in fat-1 and WT cells. Reactive oxygen intermediates were estimated by FACS, as described in the Materials and Methods. C, Representative density plots of reduced respiratory burst in infected fat-1 macrophages. The analysis was restricted to the cell population in density plots and the region R2 was created for events with high intensity of red dihydroethidium (EB) fluorescence. The percentage of events in that region was determined by CellQuest. The basal level of oxidized EB due to metabolic activity of control uninfected cells was used to set gates. Data are representative of 3 independent experiments. D, Quantitative data show the relative percentage of EB red fluorescence. *P < .001; **P < .01.
One h postinfection, cells from fat-1 mice had significantly (P < .01) lower levels of reactive oxygen (mean level ± SEM, 20.78% ± 3.12%) than those from wild-type animals (mean level ± SEM, 38.77% ± 4.09%) (Figure 5C). A trend toward reduced NO levels was observed at 24 and 48 h postinfection, but the differences were not statistically significant (data not shown).
DISCUSSION
Our data show that fat-1 mice have an increased susceptibility to pulmonary M. tuberculosis infection, as demonstrated by higher bacillary loads (Figure 1) and poorly organized inflammatory responses in lungs (Figure 2), compared with infected wild-type littermates. This is the first report to address the role of n-3 PUFAs in tuberculosis with use of the fat-1 genetic model as an alternative to diet manipulation. Our observation that endogenous n-3 PUFA enrichment in mice increases susceptibility to tuberculosis is complementary to our previous study that used dietary intervention in guinea pigs [4]. We showed that n-3 PUFA–fed guinea pigs infected under conditions identical to those used in the current study had defective bacterial clearance in lungs at 3 and 6 weeks postinfection [4]. The fact that the same detrimental n-3 PUFA effect on pulmonary tuberculosis was observed in 2 different animal species (mouse and guinea pig) following fatty acid manipulation by 2 different means (genetic vs diet) increases our confidence on the validity and generalizability of the results. Indeed, it has been reported that n-3 PUFA dietary supplementation can impair the host resistance to other intracellular pathogens [37, 38].
In contrast, Jordao et al [5] recently reported a beneficial effect of n-3 PUFAs in M. tuberculosis–infected mice. Such contrasting results could be explained by the different genetic back- ground of the mice (C57BL/6 vs BALB/c) or by procedural differences (eg, route of infection, inoculums, or use of dietary vs genetic approach). The fatty acid levels in fat-1 mice are comparable to those in animals fed a high n-3 PUFA diet [24], indicating that this model mimics the changes in fatty acid composition observed in dietary studies. Animals appeared to be normal and healthy with no additional phenotypic changes other than the n-3 PUFA enrichment. Differences in n-3 PUFAs that are endogenously produced and those obtained exogenously from diet in terms of acylation of signaling proteins or activation of receptor-mediated signaling pathways are unknown.
Statistically significant increased bacterial loads in fat-1 mice were observed in both lungs and spleens; however, it remains to be determined whether the modest magnitude of the differences found in this study has any biological relevance in humans. Because well-organized tuberculous granulomas help to contain bacterial dissemination, the increase bacterial growth in fat-1 mice could be attributed to the deficient formation of functional mature granuloma-like lesion. Reduced TNF-α levels in fat-1 mice could be a hypothetical explanation, because this cytokine plays a pivotal role in granuloma development [40]. Changes in cytokine production in vivo in infected lungs remain to be determined.
This transgenic model gave us the opportunity to address one of the potential mechanisms by which n-3 PUFAs could alter the resistance to tuberculosis. Macrophages from fat-1 mice infected ex vivo had defective cell activation characterized by reduced levels of proinflammatory cytokines (Figure 4). Reduced levels of TNF-α, IL-1β, and IL-6 have been reported before in fat-1 mice [39, 41] and dietary studies [42] [5, 43]. Thioglycollate-elicited macrophages represent a population of circulating monocytes recruited and activated in response to a stimulus, which mimics the arrival of inflammatory cells to the site of infection. However, it remains to be established whether more relevant populations, such as alveolar or lung macrophages, resemble these findings.
We and other groups [3, 5, 11, 12] have shown that n-3 PUFAs favors bacterial survival in M. tuberculosis–infected macrophages in vitro, in agreement with our ex vivo bacterial load observations. The difference in bacterial loads ex vivo seems to be explained by differences in the starting bacterial burden at 1 h. Our data are consistent with previous reports in which dietary n-3 PUFAs reduced phagosomal maturation [3] (D.L.B., L. Ly, Y.-Y.F., R.S.C., and D.N.M., unpublished data); however, because only one time point was evaluated, transient or delayed recruitment of lysotracker in cells from fat-1 mice cannot be ruled out. The finding of a reduced oxidative burst in response the M. tuberculosis infection in cells from fat-1 mice (Figure 5) is supported by previous studies in which fish oil decreased not only the oxidative burst in Kupffer cells infected with Salmonella [44] but also the nitric oxide response in fat-1 mice [20].
Defects in activation, recruitment, and functionality of other cellular components of the immune response could account for the reduced resistance to tuberculosis observed in fat-1 mice. n-3 PUFAs reduced T cell activation, proliferation and Th1 cytokine generation [13, 14], and antigen presentation in dendritic cells [45]. Therefore, changes in other cellular functions, including autophagy, chemokine production, and eicosanoid generation could also contribute to the observed phenotype [19, 21, 46]. It is possible that the differences observed might be explained by loss of n-6 PUFAs and their known proinflammatory effects (Table 1).
Current interest in n-3 PUFAs focuses on their protective properties against a wide range of inflammatory and autoimmune diseases. However, we observed that genetically enhanced levels of n-3 PUFAs increase the susceptibility to tuberculosis in fat-1 mice, in conjunction with deficiency in several inflammatory macrophage functions. Additional experiments are necessary to gain better understanding of how n-3 PUFAs increase susceptibility to infection and to assess the risk of n-3 consumption in humans, especially at-risk populations in areas where tuberculosis is endemic. The long-term goal would be to provide information on which to base public dietary advisories, to prevent any detrimental effects of dietary n-3 PUFAs.
Acknowledgments
We thank Evelyn Callaway for the mouse breeding and technical support. Dr Brad Weeks, a board certified veterinary pathologist in the Department of Veterinary Pathobiology, Texas A&M University, performed the histo-pathological assessment of infected tisses. Technical assistance was also provided by Lan Ly, Lin Bustamante, Jane Miller, Quian Jia, Ammini Jeevan, Veronica Sanchez, Selva Kumar and Christine McFarland. We thank Vernon Tesh and James Samuel for their valuable comments and suggestions. Scott Franzblau donated the GFP expressing (Ace::GFP) M. tuberculosis H37Rv. Karen Dobos-Elder provided the E193 whole cell lysate rabbit polyclonal, through the National Institutes of Health Tuberculosis Vaccine Testing and Research Materials Contract to Colorado State University.
Financial support: USPHS (AI-15495, CA-129444, DK-071707) and the National Institutes of Health (P30-ES09106).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: Keystone Tuberculosis Meeting, November 2008, Denver, Colorado; Texas Tuberculosis Researcher Symposium, February 2009, Houston, Texas; and 14th Annual Student Research Symposium, April 2009, College Station, Texas.
References
- 1.McMurray DN, Bartow RA, Mintzer CL. Impact of protein malnutrition on exogenous reinfection with Mycobacterium tuberculosis. Infect Immun. 1989;57:1746–1749. doi: 10.1128/iai.57.6.1746-1749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anes E, Kuhnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol. 2003;5:793–802. doi: 10.1038/ncb1036. [DOI] [PubMed] [Google Scholar]
- 4.McFarland CT, Fan YY, Chapkin RS, Weeks BR, McMurray DN. Dietary polyunsaturated fatty acids modulate resistance to Mycobacterium tuberculosis in guinea pigs. J Nutr. 2008;138:2123–2128. doi: 10.3945/jn.108.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordao L, Lengeling A, Bordat Y, et al. Effects of omega-3 and -6 fatty acids on Mycobacterium tuberculosis in macrophages and in mice. Microbes Infect. 2008;10:1379–1386. doi: 10.1016/j.micinf.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Yaqoob P. Fatty acids and the immune system: from basic science to clinical applications. Proc Nutr Soc. 2004;63:89–104. doi: 10.1079/PNS2003328. [DOI] [PubMed] [Google Scholar]
- 7.Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107:1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- 8.Dewey A, Baughan C, Dean T, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 2007;(1):CD004597. doi: 10.1002/14651858.CD004597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan GJ, Fraser RI, Comstock GW. Tuberculosis in Alaska, 1970. The continued decline of the tuberculosis epidemic. Am Rev Respir Dis 1972. 105:920–926. doi: 10.1164/arrd.1972.105.6.920. [DOI] [PubMed] [Google Scholar]
- 11.Paul KP, Leichsenring M, Pfisterer M, et al. Influence of n-6 and n-3 polyunsaturated fatty acids on the resistance to experimental tuberculosis. Metabolism. 1997;46:619–624. doi: 10.1016/s0026-0495(97)90003-2. [DOI] [PubMed] [Google Scholar]
- 12.Mayatepek E, Paul K, Leichsenring M, et al. Influence of dietary (n-3)-polyunsaturated fatty acids on leukotriene B4 and prostaglandin E2 synthesis and course of experimental tuberculosis in guinea pigs. Infection. 1994;22:106–112. doi: 10.1007/BF01739016. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Kim W, Zhou L, et al. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–2398. doi: 10.1093/jn/136.9.2391. [DOI] [PubMed] [Google Scholar]
- 14.Arrington JL, Chapkin RS, Switzer KC, Morris JS, McMurray DN. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin Exp Immunol. 2001;125:499–507. doi: 10.1046/j.1365-2249.2001.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ cells by affecting membrane raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyun-saturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 18.Hudert CA, Weylandt KH, Lu Y, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia S, Lu Y, Wang J, et al. Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci U S A. 2006;103:12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak J, Weylandt KH, Habbel P, et al. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007;28:1991–1995. doi: 10.1093/carcin/bgm166. [DOI] [PubMed] [Google Scholar]
- 21.Jia Q, Lupton JR, Smith R, et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berquin IM, Min Y, Wu R, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmocker C, Weylandt KH, Kahlke L, et al. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology. 2007;45:864–869. doi: 10.1002/hep.21626. [DOI] [PubMed] [Google Scholar]
- 24.Lau BY, Ward WE, Kang JX, Ma DW. Femur EPA and DHA are correlated with femur biomechanical strength in young fat-1 mice. J Nutr Biochem. 2009;20:453–461. doi: 10.1016/j.jnutbio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Grover AA, Kim HK, Wiegeshaus EH, Smith DW. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at –70 C. J Bacteriol. 1967;94:832–835. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawant KV, McMurray DN. Guinea pig neutrophils infected with Mycobacterium tuberculosis produce cytokines which activate alveolar macrophages in noncontact cultures. Infect Immun. 2007;75:1870–1877. doi: 10.1128/IAI.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiegeshaus EH, McMurray DN, Grover AA, Harding GE, Smith DW. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970;102:422–429. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T, Lasco TM, Uchida K, et al. Mycobacterium bovis BCG vaccination modulates TNF-alpha production after pulmonary challenge with virulent Mycobacterium tuberculosis in guinea pigs. Tuberculosis (Edinb) 2007;87:155–165. doi: 10.1016/j.tube.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Lasco TM, Cassone L, Kamohara H, Yoshimura T, McMurray DN. Evaluating the role of tumor necrosis factor-alpha in experimental pulmonary tuberculosis in the guinea pig. Tuberculosis (Edinb) 2005;85:245–258. doi: 10.1016/j.tube.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Elsinghorst EA. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 31.Folch J LM, Sloane GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 32.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapkin RS, Akoh CC, Miller CC. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J Lipid Res. 1991;32:1205–1213. [PubMed] [Google Scholar]
- 34.Changsen C, Franzblau SG, Palittapongarnpim P. Improved green fluorescent protein reporter gene-based microplate screening for antituberculosis compounds by utilizing an acetamidase promoter. Antimicrob Agents Chemother. 2003;47:3682–3687. doi: 10.1128/AAC.47.12.3682-3687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994;55:253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- 36.Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 37.Fritsche KL, Shahbazian LM, Feng C, Berg JN. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin Sci (Lond) 1997;92:95–101. doi: 10.1042/cs0920095. [DOI] [PubMed] [Google Scholar]
- 38.Irons R, Anderson MJ, Zhang M, Fritsche KL. Dietary fish oil impairs primary host resistance against Listeria monocytogenes more than the immunological memory response. J Nutr. 2003;133:1163–1169. doi: 10.1093/jn/133.4.1163. [DOI] [PubMed] [Google Scholar]
- 39.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 40.Bean AG, Roach DR, Briscoe H, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 41.Bhattacharya A, Chandrasekar B, Rahman MM, Banu J, Kang JX, Fernandes G. Inhibition of inflammatory response in transgenic fat-1 mice on a calorie-restricted diet. Biochem Biophys Res Commun. 2006;349:925–930. doi: 10.1016/j.bbrc.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 42.Kesavalu L, Bakthavatchalu V, Rahman MM, et al. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol Immunol. 2007;22:232–239. doi: 10.1111/j.1399-302X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 43.Robinson DR, Urakaze M, Huang R, et al. Dietary marine lipids suppress continuous expression of interleukin-1 beta gene transcription. Lipids. 1996;31(Suppl):S23–S31. doi: 10.1007/BF02637046. [DOI] [PubMed] [Google Scholar]
- 44.Eicher SD, McVey DS. Dietary modulation of Kupffer cell and splenocyte function during a Salmonella typhimurium challenge in mice. J Leukoc Biol. 1995;58:32–39. doi: 10.1002/jlb.58.1.32. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Hao Q, Li QR, et al. Omega-3 polyunsaturated fatty acids affect lipopolysaccharide-induced maturation of dendritic cells through mitogen-activated protein kinases p38. Nutrition. 2007;23:474–482. doi: 10.1016/j.nut.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Mancuso P, Whelan J, DeMichele SJ, Snider CC, Guszcza JA, Karlstad MD. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit Care Med. 1997;25:1198–1206. doi: 10.1097/00003246-199707000-00023. [DOI] [PubMed] [Google Scholar]