Abstract
Reproducible neurophysiological testing paradigms are critical to multi-center studies of neuropathy associated with impaired glucose regulation (IGR), yet the best methodologies and endpoints remain to be established. This study evaluates the reproducibility of neurophysiologic tests within a multi-center research setting. Twenty-three participants with neuropathy and IGR were studied. Reproducibility of QSART and QST (using the CASE IV system) were determined in a subset of patients at two sessions and calculated from intraclass correlation coefficients (ICC). QST (cold detection threshold ICC 0.80, vibration detection threshold ICC 0.75) was more reproducible than QSART (ICC foot 0.52). Performing multiple tests in one setting did not improve reproducibility of QST. QST reproducibility in IGR patients is similar to other patient groups studied. QSART reproducibility was significantly lower than QST. In this group of patients, the reproducibility of QSART was unacceptable for a secondary endpoint measure in clinical research trials.
Keywords: neuropathy, impaired glucose tolerance, clinical trials, QSART, QST
Introduction
A need exists for accurate and early diagnosis of neuropathies in clinical trials where participants have impaired glucose regulation (impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT)). Neuropathy trials require screening tests that are sensitive, specific, can be performed without highly specialized training, and are standardized across multiple study sites. The ideal test would allow rapid and reproducible screening of potential participants. When used as a primary or secondary endpoint measure in longitudinal studies, the test needs to be reproducible to ensure that variations in results are due solely to changes in nerve physiology. A further problem facing investigators in large clinical neuropathy trials is the choice of an appropriate primary endpoint measure. Electrophysiological tests offer the advantage of early pre-clinical diagnosis, and thus of early intervention to prevent neuropathy. However, nerve conduction studies (NCS), which are the most commonly used electrophysiological tests, do not evaluate small fiber function and thus may fail to identify patients with this type of neuropathy.
Quantitative sensory testing (QST) and quantitative sudomotor axon reflex testing (QSART) have both been studied in large single center studies of diabetes (1-3). Within the expertise of a single site, QST has been shown to provide highly reproducible within-test and between-test results (4-8). QST has been used as a secondary endpoint in a number of clinical studies evaluating therapeutic outcomes, for example HIV-related and diabetic neuropathies (9-11) and QSART has been used as a confirmatory test of neuropathy in several small studies of small fiber neuropathy (12-15). The reliability and reproducibility of QSART has not been evaluated in a multicenter trial, nor has its reproducibility been compared to other measures of small fiber function. Evaluation of the reproducibility of these tests in the setting of a multicenter clinical trial is important in promoting effective use of neurophysiologic tests as surrogate endpoint measures of neuropathy in future clinical trials.
Research Design and Methods
Study Design
Participants enrolled in a non-randomized multicenter longitudinal cohort study evaluating early neuropathy associated with IGR (as part of the Impaired Glucose Tolerance Causes Neuropathy Study, NIH NS38849) were evaluated with nerve conduction studies, QST [vibration detection threshold (VDT) and cold detection threshold (CDT)], QSART, and intraepidermal nerve fiber density (IENFD) through skin biopsy as part of the study design. The criteria for inclusion within the study were IGR confirmed on at least two separate occasions, signs and symptoms of peripheral neuropathy, and an abnormality in at least one of the following: nerve conduction studies (NCS), QST, or QSART (16). Symptoms and signs of neuropathy included numbness, paresthesias, and/or pain in the extremities in a stocking/glove distribution, absent or reduced reflexes, decreased perception of vibratory, sharp stimuli, or decreased proprioception on examination. IGR was defined according to the WHO and ADA definition at that time; fasting glucose ≥110 mg/dL and <126 mg/dL and/or 2 hour glucose ≥140 mg/dL and <200 mg/dL (17). IENFD was not chosen as an initial screening measure because one purpose of the study was to pilot its use as an endpoint, and to allow entry based on its abnormality would have biased the results in its favor. Subjects were excluded from the study if other causes of neuropathy existed; including concurrent use of neuropathy-inducing medication, environmental toxins, hereditary neuropathy, and abnormal laboratory screening results (thyroid stimulating hormone, serum protein electrophoresis and immunofixation, antinuclear antibody, vitamin B12 levels, folate levels, and erythrocyte sedimentation rate). Subjects were consented according to the Declaration of Helsinki. Studies were performed sequentially on participants from two study sites, the University of Michigan and the University of Utah. Reproducibility testing for QST and QSART was performed on two separate days, separated by at least one day to one month in a subset of 23 patients.
Of the 23 total patients included, 19 patients underwent repeat QST testing, and 13 patients underwent repeat QSART testing. 9 patients had both QSART and QST repeat testing, 10 patients had only repeat QST testing and 4 patients had only repeat QSART testing. This was due to subject availability for repeat testing, therefore, not all subjects were able to have both QST and QSART repeat testing performed. These 23 patients were not significantly different than the rest of the cohort evaluated and were the initial patients enrolled in the IGT cohort.
QST
QST was performed on the left dorsal foot using the computer-aided sensory evaluator system (CASE IV; Stillwater, MN) and previously published methodology (18;19) . Vibration and cold detection thresholds (VDT and CDT) were tested consecutively in each patient. Subjects received 3 tests of both VDT and CDT on day one (trial 1) and day 2 (trial 2). Three tests were performed at each session to determine if frequent testing improved the reproducibility of results. The examiner read the instructions at the beginning of each trial and ensured the participant fully understood the instructions. Conditions of testing were standardized and remained identical between individual tests and trials.
QSART
QSART was performed only once on each day because residual sweat volume and local skin alterations can cause variability of results (20). Skin was prepped with acetone, isopropyl alcohol, and water. Capsules were placed at the medial forearm (75% of the distance from the ulnar epicondyle to the pisiform bone), the proximal leg (lateral aspect, 5 cm distal to the fibular head), medial distal leg (5 cm proximal to the medial malleolus), and proximal foot (over the extensor digitorum brevis muscle). 10% acetylcholine was iontophoresed for 5 minutes. The sweat response was recorded over 10 minutes (5 minutes of ionotophoresis plus 5 minutes for sweat volumes to return to baseline). QSART and CASE IV equipment was purchased from WR Electronics, Stillwater, MN, and are similar but not identical to original equipment designed at the Mayo Clinic and used in clinical trials at that center.
Statistical Design
Intraclass correlation coefficients (ICC) were calculated to investigate the reliability of the QST and QSART scores. Each test was considered to be a random sample of all possible tests so that the results could be generalized (21). SAS macro %intracc (http://support.sas.com/kb/25/031.html) was used to calculate ICCs. Because of the non-linear aspect of patient sensation expressed in just noticeable difference (JND) values, the normal deviate was also used for analysis of QST results, but there was no significant difference in ICC values calculated from JND or normal deviates. CDT and VDT were analyzed separately. ICC for QSART results were calculated using both raw sweat volumes and standard normal deviates (SND). Standard normal deviates (z score) for QSART sweat volumes were adjusted to compare to previously published normative data (22), but this did not produce significantly different results, so ICC results reported are from raw sweat volumes.
Analyses were performed using SAS Version 9.1.3 (SAS Institute Inc. Cary, NC) and R Version 2.3.1.
Results
For analysis of the reproducibility of neurophysiologic tests, twenty-three subjects were prospectively included in the dataset. The average age of the cohort was 57.6 years (range 45-74 years); 7 were male and 16 female; 1 African American, 2 Hispanic, and the remainder Caucasian. The mean BMI was 32.7 (range 25.3-38.7). HbA1C levels fell within the normal range on tested participants. Patients had an average length of 60 months of neuropathy before being enrolled in the study (range 12-180). The average fasting glucose was 99, 2-hour glucose was 166 mg/dL (142-199 mg/dL). 19 participants of 23 participated in QST reproducibility testing, 13 participants of 23 completed QSART in both sessions. 9 participants of the 23 completed both QST and QSART and are included in both results.
Reproducibility of CDT and VDT Results
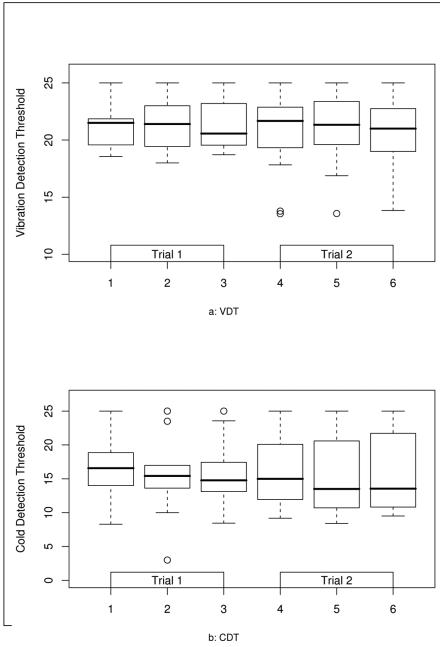
Comparison of Test 1 (first test of Trial 1) and Test 4 (first test of Trial 2) yielded an ICC of 0.81 for CDT (Table 1). Utilizing the means of tests on Trial 1 and Trial 2 did not improve reliability significantly (ICC 0.83). Reproducibility between tests within each trial is shown in Fig. 1A, and was not as strong; ICC for Test 1 vs. 2 (0.83) vs. 1-3 was 0.85, thus there is no improvement in reliability between trial 2 and 3 compared to 1 and 2. VDT reproducibility was similarly strong between trials. The ICC for VDT Test 1 and Test 4 was 0.74 (Table 1). Results were not significantly different for means of VDT tests of Trial 1 and Trial 2 (ICC 0.82) as indicated in Fig. 1B. Reproducibility of individual tests performed on the same day was significantly lower for VDT than CDT (0.82 vs. 0.55). One patient performed with more variability on the second day, which may have led to the significantly lower ICC mean of trials for VDT.
Table 1.
CDT and VDT Reproducibility: Test 1 vs. Test 4 and Means of Trial 1 vs. Trial 2
| Measure | CDT Test 1 | CDT Test 4 | VDT Test 1 | VDT Test 4 |
| Mean JND | 16.9 ± 4.73 | 16.20 ± 5.17 | 21.08 ± 1.88 | 20.42 ± 3.62 |
| Approximate Change from Baseline | −7.3 | −5.2 | 172.5 | 120 |
| (°C for CDT or μm for VDT ) | ||||
| ICC | 0.80 | 0.80 | 0.75 | 0.75 |
|
| ||||
| Measure | CDT | CDT | VDT | VDT |
| MeanTrial 1 | MeanTrial 2 | MeanTrial 1 | MeanTrial 2 | |
| Mean JND | 16.23 ± 5.18 | 15.85 ± 5.63 | 21.32 ± 1.94 | 20.64 ± 3.45 |
| Approximate Change from Baseline | −5.3 | −4.5 | 172 | 127 |
| (°C for CDT or μm for VDT ) | ||||
| ICC | 0.83 | 0.83 | 0.55 | 0.55 |
JND: Just noticeable difference: discrete magnitudes of stimulation by the CASE IV device exponential algorithmic function; CDT, cold detection threshold; VDT, vibration detection threshold; ICC, intra-class correlation coefficient. Approximate change from baseline is mean of minimum degrees of temperature/ μm vibration required for patients to perceive stimulus from baseline temperature/ vibration at beginning of CASE IV testing paradigm.
Figure 1.
A. Reproducibility of VDT between individual tests (T1-6) and trials in subjects with impaired glucose tolerance and small fiber neuropathy. The box plot indicates the mean, 25th and 75th percentiles for VDT expressed in JND units for each test.
B. Reproducibility of CDT between individual tests (T1-6) and trials in subjects with IGR and neuropathy. The box plot indicates the mean, 25th and 75th percentiles for CDT results expressed in JND units for each test.
Reproducibility of QSART Results
No patient had a lack of sweat response at all four sites. Most participants had a proximal to distal gradient, with higher sweat volumes proximally. Reproducibility was similar for the forearm, proximal leg and foot sites (0.52-0.63, Table 2). The distal leg site was the least reproducible, with an ICC of 0.42. One patient had a severe leak at the forearm and proximal leg sites which made it impossible to accurately determine the volume at these sites and the data was excluded from the analysis.
Table 2.
Quantitative Sudomotor Axon Reflex Test (QSART): Means of Trial 1 vs. Trial 2
| Measure (μl) | Trial 1 | Trial 2 | ICC |
|---|---|---|---|
| Mean sweat volume at the foot | 0.76 ± 0.31 | 0.76 ± 0.19 | 0.52 |
| Mean sweat volume at the distal leg | 1.24 ± 0.31 | 1.83 ± 0.74 | 0.42 |
| Mean sweat volume at the proximal leg | 0.76 ± 0.19 | 0.98 ± 0.20 | 0.63 |
| Mean sweat volume at the forearm | 1.17 ± 1.35 | 0.73 ± 0.73 | 0.55 |
ICC, intra-class correlation coefficient
Discussion
Neuropathy occurs over time in more than half of diabetic patients (23;24). Nerve conduction studies demonstrate that neuropathy is already present in 10-18% of patients at the time of diabetes diagnosis (25;26), suggesting that peripheral nerve injury occurs at early stages of disease and with milder glycemic dysregulation. Neuropathy occurring early in diabetes is usually characterized by symmetrical sensory symptoms including pain, and autonomic dysfunction (27-32). In the largest prospective series, 81% of neuropathy patients with IGT had exclusively sensory complaints, and 92% recognized neuropathic pain as a dominant symptom of their neuropathy (33). Small myelinated and unmyelinated fibers convey sensations of light touch, pain and temperature (34;35), whereas large fiber loss results in decreased vibratory sensation, joint position sense, and recorded sural nerve sensory action potentials (19;36). Pain symptoms and impaired temperature perception in IGT neuropathy suggest prominent early involvement of the small unmyelinated nerve fibers that mediate pain, temperature sensation and autonomic function. However, the present study indicates that in IGT neuropathy, as in diabetic neuropathy, large myelinated fibers are also affected as determined by an abnormal VDT in up to 72 % of participants (using the 95th percentile) and abnormal sural nerve amplitude in 76% of participants (37). This indicates that there is significant involvement of distal large fiber function even before a patient converts from IGT or IFG to diabetes. Furthermore, these findings support the concept that tests of large fiber function should be included in trial entry criteria for early diabetic neuropathy.
Identification of ideal endpoints of neuropathy has been controversial. Recently, a case definition of distal symmetrical polyneuropathy has been developed to standardize clinical research (38). This report concluded that simple composite examination scores were as accurate as more complex examinations. However, QST and autonomic tests were not included in the recommendations because of concerns about the variability of QST or the lack of availability of autonomic testing in medical centers.
Reproducibility results for QST using the CASE IV system in this multi-center study are similar to those reported in other multicenter evaluations (39;40). The equipment used in this multicenter study is now available in many medical centers conducting neuropathy trials and is familiar to diabetic neuropathy trialists.
QST can measure multiple stimuli thresholds, but has the drawback of being a biopsychophysical test, and it has been argued that QST is limited by the subject’s motivation and concentration during testing (41). Despite provisions made in Case IV QST testing for such an eventuality, a subject may consciously influence results of the test making QST unreliable (42;43). However, in clinical trials where participants are motivated to closely follow the testing protocol, and where such a profound conscious bias is lacking, this study indicates that QST would be a reliable test.
There are multiple QST methodologies which are commercially available. Most QST testing uses either the method of limits or the method of levels. The method of limits uses a continuously increasing or decreasing stimulus and the patient reports when they feel the stimulus, or the stimulus is absent. This method (because it depends on subject reaction time) is dependent on patient motor abilities and attention. The method of levels (“forced choice”) utilized in the Case IV algorithm uses a series of predefined amounts of stimulus and the patient reports whether they perceive the sensation. The stimulus is increased or decreased based on the patient’s choice. This method is more time consuming, but may be more accurate as it does not depend on reaction time of the patient. If a patient makes a choice twice which is incompatible with previous choices, the testing stops.
Other QST systems have been evaluated for reliability including the vibraton, neurothesiometer, biothesiometer, the Rydel-Seiffer tuning fork for vibration perception thresholds, Medoc, and the Marstock device, among others. However, comparison between instruments is difficult due to multiple statistical analyses used for evaluation of variability. The neurothesiometer and Reidel-Seiffer tuning fork have also been shown to have significant reproducibility, with coefficients of variance of 8-13% and 4% respectively (44;45). Reliability of thermal thresholds appears to be less than for vibration perception thresholds using the Medoc device but was not seen in this study utilizing the CASE IV system (46). QST testing may also be more reliable than current perception threshold (CPT) testing (46).
In our study, we compared variability between separate days using both a single test (the first test on each day) and mean of three tests performed on the same day, as well as variability between tests performed on the same day. Both CDT and VDT testing were performed similarly, comparing the first trial on each session as well as the mean of each session. There was a significant difference between the two tests in intra-trial and intra-session variability as VDT performed less well using the mean of each session. This may have been due to the small sample size. Results otherwise did not improve or decrease in reliability between test 1 and 2, and between test 2 and 3. This suggests that subject conditioning or distractibility were not consistent factors causing changes in results between individual tests. The relatively high reproducibility obtained in this multicenter study will increase the statistical power for QST as an endpoint measure compared to other less reproducible electrophysiological measures. However, these results, especially for VDT, suggest that multiple tests at one session may be deleterious towards reproducibility.
QSART is an objective measure of postganglionic sudomotor function, which is typically involved in small fiber neuropathies and autonomic neuropathies (47-49). Unlike QST, QSART permits comparison of distal to proximal sweat responses and thus assessment of length-dependent neuropathies. QSART has relatively good sensitivity in this population (50) and is also abnormal in patients with IGR without defined neuropathy compared to normoglycemic patients, suggesting sensitivity in identifying preclinical neuropathy in this population (51). However, QSART reproducibility was significantly lower than that for QST, mitigating its usefulness as a longitudinal measure in future research trials.
The ICC values for QSART obtained in this study indicate that reproducibility of QSART may be inadequate in a large clinical trial. This is most likely due to the greater number of variables that can affect QSART results such as skin temperature, the concentration and purity of acetylcholine solution used, possible air bubbles, ineffective skin preparation preventing contact with acetylcholine, variable skin impedance, or possible variability in the stimulator output. These variables may have caused the low reproducibility reported in this study. In addition, the commercially available QSWEAT device was used at both sites, whereas previous reproducibility data for QSART was performed at Mayo Clinic with similar but not identical equipment. The differences between the two devices may also have contributed to the decreased reproducibility observed. However, the current study clearly indicates that future multicenter studies using available equipment should expect only moderate reproducibility in the QSART and this will significantly reduce the statistical power of QSART as an endpoint measure.
IENFD is more sensitive in detecting early diabetic neuropathy than electrophysiological tests and maintains excellent reproducibility evaluating biopsies by separate readers (ICC=0.98), and between punches (at the same site; r=0.87) (52-58). The advantage of continuing to use electrophysiological and psychophysical tests is that they are non-invasive, commonly available in many clinical trial centers, and provide rapid assessment of neuropathy based on standardized approaches to diagnosis. QST is a highly reproducible test when used in a multicenter trial, and reproducibility was similar in both participating sites in this study, whereas this was not true for QSART.
The main limitation of this study was the small sample size. However, as mentioned earlier, the similar results obtained for QST testing to other reported studies for intra-trial reproducibility suggest that this was not a significant factor. However, the significantly low intra-session reproducibility and lower reproducibility of the mean of session 1 vs. session 2 may be due to the small sample size. A benefit of this study was that the studies were performed by one technician at each site. Results were available to investigators between each session, which may have introduced bias as they were not blinded to the results.
In conclusion, QST using the CASE IV device would be an appropriate screening tool and endpoint measure for multi-center trials of neuropathy in IGR. QSART, using the QSWEAT device, has only moderate reproducibility which mitigates its use as an endpoint measure.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Phillip Low, Mayo Clinic, Rochester, MN for assistance with analysis of the QSART data, Ms. Susan Nalepa for technical assistance during the trial Supported in part by NIH T32 NS07222 (ACP), NIH NS40458 and DK064814 (JRS, JH, GS); NIH NS40458 (JG); NIH NS40458, NS36778, NIH NS38849, PFUND, and JDRF (ELF); NIH NS40458, NS42056, JDRF, Office of Research Development (Medical Research Service), Department of Veterans Affairs, ADA (JWR); MO1-RR00042 (Michigan GCRC); DK02-016 (Utah GCRC); NIDDK #5P60DK-20572 (MDRTC).
Abbreviations
- QSART
quantitative sudomotor axon reflex test
- QST
quantitative sensory test
- CASE IV
computer-aided sensory evaluator system
- CDT
cold detection threshold
- VDT
vibration detection threshold
- NCS
nerve conduction studies
- IGT
impaired glucose tolerance
- IFG
impaired fasting glucose
- IGR
impaired glucose regulation (includes IGT, IFG)
- ICC
intra-class correlation coefficient
- HIV
human immunodeficiency virus
- IENFD
intraepidermal nerve fiber density
- WHO
World Health Organization
- ADA
American Diabetes Association
- mg
milligrams
- dL
deciliters
- JND
just noticeable difference, discrete magnitudes of stimulation by the CASE IV device exponential algorithmic function used to determine threshold of perception using CASE IV
- SND
standard normal deviate
Reference List
- 1.Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997 Jul;49(1):229–39. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- 2.Dyck PJ, Karnes JL, O'Brien PC, Litchy WJ, Low PA, Melton LJ., III The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology. 1992 Jun;42(6):1164–70. doi: 10.1212/wnl.42.6.1164. [DOI] [PubMed] [Google Scholar]
- 3.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997 Dec;20(12):1561–8. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997 Jul;49(1):229–39. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- 5.Dyck PJ, Karnes JL, O'Brien PC, Litchy WJ, Low PA, Melton LJ., III The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology. 1992 Jun;42(6):1164–70. doi: 10.1212/wnl.42.6.1164. [DOI] [PubMed] [Google Scholar]
- 6.Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993 Aug;43(8):1508–12. doi: 10.1212/wnl.43.8.1508. [DOI] [PubMed] [Google Scholar]
- 7.Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, O'Brien PC. Cool, warm, and heat-pain detection thresholds: testing methods and inferences about anatomic distribution of receptors. Neurology. 1993 Aug;43(8):1500–8. doi: 10.1212/wnl.43.8.1500. [DOI] [PubMed] [Google Scholar]
- 8.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983 Nov;14(5):573–80. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 9.Schifitto G, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, et al. Long-term treatment with recombinant nerve growth factor for HIV-associated sensory neuropathy. Neurology. 2001 Oct 9;57(7):1313–6. doi: 10.1212/wnl.57.7.1313. [DOI] [PubMed] [Google Scholar]
- 10.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993 Apr;43(4):817–24. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 11.Apfel SC, Schwartz S, Adornato BT, Freeman R, Biton V, Rendell M, et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000 Nov 1;284(17):2215–21. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- 12.Kihara M, Mitsui M, Nishikawa S, Nishimoto K, Takahashi M. Comparison of electrophysiologic and autonomic tests in sensory diabetic neuropathy. Clin Auton Res. 1998 Aug;8(4):213–20. doi: 10.1007/BF02267784. [DOI] [PubMed] [Google Scholar]
- 13.Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology. 1999 Nov 10;53(8):1641–7. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 14.Novak V, Kanard R, Kissel JT, Mendell JR. Treatment of painful sensory neuropathy with tiagabine: a pilot study. Clin Auton Res. 2001 Dec;11(6):357–61. doi: 10.1007/BF02292767. [DOI] [PubMed] [Google Scholar]
- 15.Low PA, Opfer-Gehrking TL, Dyck PJ, Litchy WJ, O'Brien PC. Double-blind, placebo-controlled study of the application of capsaicin cream in chronic distal painful polyneuropathy. Pain. 1995 Aug;62(2):163–8. doi: 10.1016/0304-3959(94)00261-C. [DOI] [PubMed] [Google Scholar]
- 16.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 17. Ref Type: Generic. [Google Scholar]
- 18.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993 Apr;43(4):817–24. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 19.Russell JW. Quantitative Sensory Testing. In: Bromberg M, Smith AG, editors. Handbook of Peripheral Neuropathy. Taylor and Francis LLC; 2005. pp. 45–52. [Google Scholar]
- 20.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983 Nov;14(5):573–80. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychology Bulletin. 2008;86:420–7. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983 Nov;14(5):573–80. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler D, Gries FA, Spuler M, Lessmann F. The epidemiology of diabetic neuropathy. Diabetic Cardiovascular Autonomic Neuropathy Multicenter Study Group. J Diabetes Complications. 1992 Jan;6(1):49–57. doi: 10.1016/1056-8727(92)90049-q. [DOI] [PubMed] [Google Scholar]
- 24.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993 Apr;43(4):817–24. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 25.Lehtinen JM, Niskanen L, Hyvonen K, Siitonen O, Uusitupa M. Nerve function and its determinants in patients with newly-diagnosed type 2 (non-insulin-dependent) diabetes mellitus and in control subjects--a 5-year follow-up. Diabetologia. 1993 Jan;36(1):68–72. doi: 10.1007/BF00399096. [DOI] [PubMed] [Google Scholar]
- 26.Cohen JA, Jeffers BW, Faldut D, Marcoux M, Schrier RW. Risks for sensorimotor peripheral neuropathy and autonomic neuropathy in non-insulin-dependent diabetes mellitus (NIDDM) Muscle Nerve. 1998 Jan;21(1):72–80. doi: 10.1002/(sici)1097-4598(199801)21:1<72::aid-mus10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve. 2001 Sep;24(9):1225–8. doi: 10.1002/mus.1136. [DOI] [PubMed] [Google Scholar]
- 28.Russell JW, Feldman EL. Impaired glucose tolerance--does it cause neuropathy? Muscle Nerve. 2001 Sep;24(9):1109–12. doi: 10.1002/mus.1122. [DOI] [PubMed] [Google Scholar]
- 29.Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve. 2001 Sep;24(9):1229–31. doi: 10.1002/mus.1137. [DOI] [PubMed] [Google Scholar]
- 30.Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001 Nov 13;57(9):1701–4. doi: 10.1212/wnl.57.9.1701. [DOI] [PubMed] [Google Scholar]
- 31.Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003 Dec;52(12):2867–73. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- 32.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003 Jan 14;60(1):108–11. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- 33.Singleton JR, Smith AG, Bromberg MB. Painful sensory polyneuropathy associated with impaired glucose tolerance. Muscle Nerve. 2001 Sep;24(9):1225–8. doi: 10.1002/mus.1136. [DOI] [PubMed] [Google Scholar]
- 34.Adriaensen H, Gybels J, Handwerker HO, Van HJ. Response properties of thin myelinated (A-delta) fibers in human skin nerves. J Neurophysiol. 1983 Jan;49(1):111–22. doi: 10.1152/jn.1983.49.1.111. [DOI] [PubMed] [Google Scholar]
- 35.Hallin RG, Torebjork HE, Wiesenfeld Z. Nociceptors and warm receptors innervated by C fibres in human skin. J Neurol Neurosurg Psychiatry. 1982 Apr;45(4):313–9. doi: 10.1136/jnnp.45.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciano CA, Russell JW, Banerjee TK, Quirk JM, Scott LJ, Dambrosia JM, et al. Physiological characterization of neuropathy in Fabry's disease. Muscle Nerve. 2002 Nov;26(5):622–9. doi: 10.1002/mus.10236. [DOI] [PubMed] [Google Scholar]
- 37.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006 Jun;29(6):1294–9. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 38.England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve. 2005 Jan;31(1):113–23. doi: 10.1002/mus.20233. [DOI] [PubMed] [Google Scholar]
- 39.Bird SJ, Brown MJ, Spino C, Watling S, Foyt HL. Value of repeated measures of nerve conduction and quantitative sensory testing in a diabetic neuropathy trial. Muscle Nerve. 2006 Aug;34(2):214–24. doi: 10.1002/mus.20577. [DOI] [PubMed] [Google Scholar]
- 40.Kincaid JC, Price KL, Jimenez MC, Skljarevski V. Correlation of vibratory quantitative sensory testing and nerve conduction studies in patients with diabetes. Muscle Nerve. 2007 Dec;36(6):821–7. doi: 10.1002/mus.20880. [DOI] [PubMed] [Google Scholar]
- 41.Freeman R, Chase KP, Risk MR. Quantitative sensory testing cannot differentiate simulated sensory loss from sensory neuropathy. Neurology. 2003 Feb 11;60(3):465–70. doi: 10.1212/wnl.60.3.465. [DOI] [PubMed] [Google Scholar]
- 42.Freeman R, Chase KP, Risk MR. Quantitative sensory testing cannot differentiate simulated sensory loss from sensory neuropathy. Neurology. 2003 Feb 11;60(3):465–70. doi: 10.1212/wnl.60.3.465. [DOI] [PubMed] [Google Scholar]
- 43.Dyck PJ, Dyck PJ, Kennedy WR, Kesserwani H, Melanson M, Ochoa J, et al. Limitations of quantitative sensory testing when patients are biased toward a bad outcome. Neurology. 1998 May;50(5):1213. doi: 10.1212/wnl.50.5.1213. [DOI] [PubMed] [Google Scholar]
- 44.Bril V, Kojic J, Ngo M, Clark K. Comparison of a neurothesiometer and vibration in measuring vibration perception thresholds and relationship to nerve conduction studies. Diabetes Care. 1997 Sep;20(9):1360–2. doi: 10.2337/diacare.20.9.1360. [DOI] [PubMed] [Google Scholar]
- 45.Kastenbauer T, Sauseng S, Brath H, Abrahamian H, Irsigler K. The value of the Rydel-Seiffer tuning fork as a predictor of diabetic polyneuropathy compared with a neurothesiometer. Diabet Med. 2004 Jun;21(6):563–7. doi: 10.1111/j.1464-5491.2004.01205.x. [DOI] [PubMed] [Google Scholar]
- 46.Lowenstein L, Jesse K, Kenton K. Comparison of perception threshold testing and thermal-vibratory testing. Muscle Nerve. 2008 Apr;37(4):514–7. doi: 10.1002/mus.20934. [DOI] [PubMed] [Google Scholar]
- 47.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983 Nov;14(5):573–80. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 48.Singer W, Spies JM, McArthur J, Low J, Griffin JW, Nickander KK, et al. Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology. 2004 Feb 24;62(4):612–8. doi: 10.1212/01.wnl.0000110313.39239.82. [DOI] [PubMed] [Google Scholar]
- 49.Reading P, Russell JW. Chronic Autonomic Neuropathies. In: Gilman S, editor. Medlink, The Information Resource for Clinical Neurology. Arbor Publishing; 2005. [Google Scholar]
- 50.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006 Jun;29(6):1294–9. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 51.Grandinetti A, Chow DC, Sletten DM, Oyama JK, Theriault AG, Schatz IJ, et al. Impaired glucose tolerance is associated with postganglionic sudomotor impairment. Clin Auton Res. 2007 Aug;17(4):231–3. doi: 10.1007/s10286-007-0426-z. [DOI] [PubMed] [Google Scholar]
- 52.Polydefkis M, Hauer P, Griffin JW, McArthur JC. Skin biopsy as a tool to assess distal small fiber innervation in diabetic neuropathy. Diabetes Technol Ther. 2001;3(1):23–8. doi: 10.1089/152091501750219994. [DOI] [PubMed] [Google Scholar]
- 53.Smith AG, Howard JR, Kroll R, Ramachandran P, Hauer P, Singleton JR, et al. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005 Jan 15;228(1):65–9. doi: 10.1016/j.jns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy WR, Wendelschafer-Crabb G. Utility of the skin biopsy method in studies of diabetic neuropathy. Electroencephalogr Clin Neurophysiol. 1999;50(Suppl):553–9. [PubMed] [Google Scholar]
- 55.Levy DM, Karanth SS, Springall DR, Polak JM. Depletion of cutaneous nerves and neuropeptides in diabetes mellitus: an immunocytochemical study. Diabetologia. 1989 Jul;32(7):427–33. doi: 10.1007/BF00271262. [DOI] [PubMed] [Google Scholar]
- 56.Lindberger M, Schroder HD, Schultzberg M, Kristensson K, Persson A, Ostman J, et al. Nerve fibre studies in skin biopsies in peripheral neuropathies. I. Immunohistochemical analysis of neuropeptides in diabetes mellitus. J Neurol Sci. 1989 Nov 93;(2-3):289–96. doi: 10.1016/0022-510x(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 57.Vlckova-Moravcova E, Bednarik J, Dusek L, Toyka KV, Sommer C. Diagnostic validity of epidermal nerve fiber densities in painful sensory neuropathies. Muscle Nerve. 2008 Jan;37(1):50–60. doi: 10.1002/mus.20889. [DOI] [PubMed] [Google Scholar]
- 58.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998 Dec;55(12):1513–20. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]