Abstract
Osteoarthritis (OA) is the most common form of joint disease and the leading cause of chronic disability in middle-aged and older populations. The development of disease-modifying therapy for OA currently faces major obstacles largely because the regulatory mechanisms for the function of joint tissue cells remain unclear. Previous studies have found that the alterations in gene expression of specific transcription factors (TFs), pro- or anti-inflammatory cytokines, matrix proteinases and extracellular matrix (ECM) proteins in articular cartilage may be involved in the development of OA. However, the regulatory mechanisms for the expression of those genes in OA chondrocytes are largely unknown. The recent advances in epigenetic studies have shed light on the importance of epigenetic regulation of gene expression in the development of OA. In this review, we summarize and discuss the recent studies on the regulatory roles of various epigenetic mechanisms in the expression of genes for specific TFs, cytokines, ECM proteins and matrix proteinases, as well the significance of these epigenetic mechanisms in the pathogenesis of OA.
Keywords: Epigenetics, Osteoarthritis, Transcription factor, Cytokine, Matrix proteinase
Introduction
Osteoarthritis (OA) is the most common form of arthritis in the U.S. and affects approximately 27 million Americans.1 As OA mainly occurs in weight-bearing joints, such as the knee and hip, OA has long been thought of as a mechanical issue.2 However, there is a growing body of evidence supporting the notion that OA is a result of the interaction between mechanical and molecular events in the affected joint.3 There is no single specific cause that has been identified for OA to date. Some risk factors, including age, gender, obesity, joint injury, genetic and mechanical abnormalities, have been shown to be associated with the development of OA.4 However, how these risk factors trigger the onset of OA still need to be elucidated. While OA is a disease of the whole joint and may affect all of the joint tissues, articular cartilage degradation is a major hallmark of OA.5 Aberrant gene expression of specific transcription factors (TFs), cytokines, matrix proteinases and extracellular matrix (ECM) structural proteins (e.g., collagens and proteoglycans) in articular chondrocytes (ACs) of human OA and animal models of OA samples has been documented. Nevertheless, the underlying regulatory mechanism for the expression of those genes in OA cartilage is not fully understood.
“Epigenetics” is referred to as changes in gene expression caused by mechanisms other than changes in the underlying DNA sequences. DNA methylation and histone modification are the two best-studied classic epigenetic regulatory mechanisms, which regulate the transcriptional activity of a cell in the nucleus. DNA methylation is a biochemical process where a methyl group is added to the cytosine or adenine, mainly at the C5 position of CpG dinucleotides, by DNA methyltransferase (DNMT). DNA hypermethylation suppresses gene transcription, while DNA hypomethylation enhances gene transcription. Histone modifications are enzymatic post-translational modifications which include methylation, acetylation, phosphorylation, sumoylation and ubiquitination.6, 7 These modifications primarily occur within the amino-terminal tails of histone proteins that regulate gene expression by changing the chromatin structure.8
A broader definition of “epigenetics” has been proposed by Egger et al as heritable changes in gene expression that are not coded in the DNA sequences.9 In this regard, non-coding RNAs (ncRNAs) which possess epigenetic-like properties have also been taken into account as one of the epigenetic mechanisms.10, 11 ncRNAs are functional RNA molecules that regulate gene expression but do not translate into proteins. ncRNAs can be mainly divided into short ncRNAs (<30 nucleotides) and long ncRNAs (lncRNAs, >200 nucleotides). Short ncRNAs include microRNAs (miRNAs), short interfering RNAs (siRNAs) and piwi-interacting RNAs (piRNAs).12 In general, miRNAs function to modify the protein expression mainly at the post-transcriptional level in cytoplasm by binding to a specific target messenger RNA (mRNA) with a complimentary sequence to induce cleavage, degradation or block translation.13 Recent progress in the study of ncRNAs has revealed the importance of ncRNAs in development and diseases.14, 15
Given the importance of epigenetics in normal development as well as cancer and age-related diseases,11 recent studies on epigenetics in OA have provided new insights into the pathogenesis of OA and new targets to develop potential therapeutic strategies for OA. In this review, we will focus on the epigenetic mechanisms for the expression of TFs, cytokines, matrix proteinases and ECM proteins in ACs, as well as their significance in the pathogenesis of OA (Table 1).
Table 1.
Gene expression changes mediated by epigenetic mechanisms in osteoarthritic chondrocytes.
| Category | Gene | Expressiona | Epigenetic regulationb |
References | ||
|---|---|---|---|---|---|---|
| DNA methylation | Histone modification | microRNA | ||||
| TFs | Nfat1 | ↓ (m) | ↔ | ✓ | ↔ | 16, 17 |
| SOX9 | ↓ (h) | ✓ | ✓ | ✓ | 18, 19, 20 | |
| Cytokines | IL-1B | ↑ (h) | ✓ | ↔ | ✓ | 21, 22, 23 |
| TNF-alpha | ↑ (h) | ↔ | ↔ | ✓ | 23 | |
| Proteinases | ADAMTS4 | ↑ (h) | ✓ | ✓ | ✓ | 24, 25, 26, 27 |
| ADAMTS5 | ↑ (h) | ✓ | ✓ | ✓ | 20, 26, 27 | |
| MMP-13 | ↑ (h) | ✓ | ✓ | ✓ | 24, 27, 28 | |
| ECM proteins | COL2A1 | ↓ (h) | ↔ | ✓ | ✓ | 20, 29, 30, 31 |
| COL9A1 | ↓ (h) | ✓ | ↔ | ↔ | 32 | |
| ACAN | ↓ (h) | ✗ | ✓ | ✓ | 20, 33 | |
Gene expression information is cited from the references of this manuscript. ↓: decrease; ↑: increase; m: mouse; h: human.
Gene expression changes are associated with specific epigenetic alterations (✓), or not (✗), or unknown (↔).
TFs
TFs are the proteins that bind to specific DNA sequences and control the transcriptional rate of the target genes from genomic DNA to mRNA, which then translate into protein in the cytoplasm. Therefore, abnormal expression of TFs has been found to be involved in the development of many diseases, including OA.
Nfat1 (NFAT1/NFATc2) is a member of the Nuclear Factor of Activated T-cells (NFAT) transcription factor family originally identified as a regulator of the expression of cytokine genes during the immune response.34, 35 NFAT1 has recently been shown to play an important role in maintaining the permanent cartilage phenotype in adult mice. Nfat1 knockout (Nfat1−/−) mice exhibit normal skeletal development, but display over-expression of numerous matrix-degrading proteinases and proinflammatory cytokines and loss of collagen-2 and aggrecan during the initiation stage of OA. These initial changes are followed by articular chondrocyte clustering, formation of chondro-osteophytes, progressive articular surface destruction, formation of subchondral bone cysts, and exposure of thickened subchondral bone.16
Our recent studies have demonstrated that NFAT1 regulates the chondrocyte function through its age-dependent expression in mouse articular cartilage. NFAT1 expression in wild-type articular chondrocytes was low in the embryonic, but high in the adult stage (2–6 months old). Our epigenetic studies17 revealed that an increase in NFAT1 expression in ACs is associated with increased H3K4me2 (a histone modification linked to transcriptional activation); while a decrease in NFAT1 expression in ACs is correlated with increased H3K9me2 (a histone modification linked to transcriptional repression). Knockdown of lysine-specific demethylase-1 (Lsd1) in embryonic ACs up-regulates NFAT1 expression concomitant with increased H3K4me2 at the Nfat1 promoter. Knockdown of Jmjc-containing histone demethylase-2a (Jhdm2a) in 6-month ACs down-regulates NFAT1 expression concomitant with increased H3K9me2 at the Nfat1 promoter. These results suggest that the age-dependent NFAT1 expression in ACs is regulated by dynamic histone methylation.17 Further study should be directed to investigate the expression of NFAT1 in aged articular cartilage and its underlying epigenetic mechanisms as well as the role of NFAT1 in the development of OA in humans.
SOX9 is a master transcription factor for chondrogenesis during the development of the skeletal system, in cooperation with SOX5 and SOX6.36, 37 Although mice with conditional postnatal deletion of Sox9 in articular cartilage did not develop OA even by the age of 18 months,38 later OA usually is associated with decreased SOX9 expression in humans.39 Kim et al recently reported that down-regulated SOX9 expression in advanced hip OA chondrocytes is mediated by DNA methylation and histone modification, including histone methylation and acetylation.18 Moreover, miRNA-145 has been identified as an inhibitor of SOX9 expression in human chondrocyte; increased miRNA-145 directly represses SOX9 expression, causing reduced expression of COL2A1 and aggrecan and an increased level of matrix metalloproteinases 13 (MMP13).19 In addition, miRNA-199a-3p and miRNA-193b has been found to down-regulate SOX9 expression.20 While SOX9 is considered a typical anabolic factor in articular cartilage, the response of cultured chondrocytes to forced expression of SOX9 has been controversial. Kypriotou et al found that overexpression of SOX9 itself was unable to restore the chondrocyte phenotype in dedifferentiated osteoarthritic chondrocytes,40 whereas Cucchiarini et al reported that r-AAV mediated SOX9 gene transfer up-regulated the expression levels of proteoglycans and type II collagen in normal and OA ACs.41 Therefore, more studies are needed to determine whether the epigenetically regulated change in SOX9 expression in articular cartilage is the cause or the result of OA.
Cytokines
Cytokines are small proteins mostly secreted by immune cells that function as signaling messengers in the immune system. It has been well-documented that cytokines play important roles in the development of rheumatoid arthritis (RA) which is a typical autoimmune disease in human joints.42 A new concept that OA is a joint disease of inflammation involving immune reaction has been proposed based on findings of the aberrant expression of cytokines in human OA and animal models of OA.43 Although many cytokines have been implicated in OA, interlukin 1-β (IL-1β) and tumor necrosis factor-α (TNF-α) are the two main proinflammatory cytokines contributing to the degradation of articular cartilage.44, 45
The epigenetic regulation of IL-1β expression in OA cartilage has been well documented. Hashimoto et al found that specific CpG sites at −299 of the IL-1β promoter has a significant impact on its promoter activity and methylation of these sites results in marked suppression of its transcriptional activity in human ACs.21 Demethylation of these sites increases the transcriptional response of IL-1β to inflammatory cytokines in human ACs.22 In addition, Santini et al found miR-149 is down-regulated in OA chondrocytes, and a functional study showed that this miRNA regulates the production of TNF-α, IL-1β and IL-6.23 Other than being regulated by epigenetic mechanisms, IL-1β also modulates epigenetic events in OA cartilage, because stimulation of OA chondrocytes with IL-1β can affect miRNA production.46 Moreover, the overall methylation status in different histological zones of human cartilage was found to be different upon IL-1β stimulation.47 Both DNA methylation and histone modification are involved in the control of TNF-α expression48; however, the epigenetic status of TNF-α in OA chondrocytes remains to be elucidated.
Matrix proteinases
The expression of matrix proteinases, including collagenases, aggrecanases and matrix metalloproteinases (MMPs), is relatively low in normal articular cartilage. Matrix proteinases are required for cartilage turnover but elevated in OA, which are deleterious factors for ECM degradation.49, 50 ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) -4 and -5 are two major aggrecanases which have been shown to play important role in development of OA.51, 52, 53, 54 MMP13, a major type II collagen-degrading collagenase, not only contributes to the onset of OA, but also contributes to irreversible joint damage during the progression of OA.55, 56 In addition to being regulated by transcription factors and cytokines, proteinase expression is also regulated by DNA methylation.24 For example, Cheung et al found that increased ADAMTS-4 expression was mediated by the loss of DNA methylation at specific CpG sites in the ADAMTS-4 promoter in OA chondrocytes.25 In OA cartilage specifically, differentially and highly expressed lncRNA-CIR not only controls the expression of collagen and aggrecan but also regulates the expression of MMP13 and ADAMTS-5.26 Moreover, several miRNAs have also been found to regulate ADAMTS-5 expression in human OA cartilage.20 In a study of cultured SW1353 chondrosarcoma cells and primary human chondrocytes, Young et al demonstrated that histone deacetylase (HDAC) inhibitors decreased the level of collagenolytic enzymes in conditioned culture medium by down-regulating the expression of MMPs and ADAMTSs, and the elevated HDAC7 expression in cartilage from OA patients was associated with up-regulated MMP13 gene expression,28 implicating that histone modification, especially acetylation, may play a role in the control of those proteinases in the pathogenesis of OA.27
Extracellular matrix proteins
Collagen and proteoglycan are the major ECM protein components of articular cartilage. The maintenance of the normal amount and architecture of these components are required for articular cartilage to fulfill its mechanical properties.57, 58 In humans, collagen gene mutations account for a family of spondyloepiphyseal dysplasias, which are associated with early-onset of OA.59 Mice that lack Col9a1 or bear a small deletion mutation in type II collagen gene display OA-like cartilage degradation.60, 61 Adult articular cartilage is an avascular tissue, in which chondrocytes, the unique cellular component, do not normally divide, but maintain low-turnover replacement of the ECM. Therefore, degeneration of articular cartilage ECM is the major feature of OA.49, 62 As discussed above, the up-regulated matrix proteinases contribute to ECM disruption. Decreased ECM synthesis activity of chondrocytes regulated by epigenetic mechanisms accounts for the loss of ECM in OA cartilage.
Histone acetyl-transferase CBP/P300 and the Class III NAD-dependent histone deacetylase Sirtuin 1 (SirT1) have been shown to co-regulate COL2A1 mRNA expression in cooperation with SOX9.29, 30 Moreover, a recent study using human chondrocytes found that histone methyltransferase Set7/9 elevated trimethylated lysine 4 on histone 3 on COL2A1 promoter, resulting in increased COL2A1 expression.31 In a study on the correlation between gene methylation and expression of aggrecan in chondrocytes, Pochl et al failed to find a significant correlation of ACAN mRNA expression levels and DNA methylation status between normal aged and osteoarthritic chondrocytes. This result suggests that DNA methylation does not play a central role in switching off ACAN promoter activity in human adult ACs.33 Although the methylation status of COL2A1 has been studied in differentiated and dedifferentiated chondrocytes,63 little is known about the role of DNA methylation in COL2A1 expression in OA chondrocytes. DNA methylation has also been found to control the decreased expression of COL9A1 mRNA in OA chondrocytes. Imagawa et al recently reported that CpG sites in COL9A1 promoter were hypermethylated and that this hypermethylated CpG attenuated SOX9 binding to the COL9A1 promoter, resulting in down-regulation of COL9A1 expression in OA cartilage.32 In addition to the regulation of SOX9 expression, miRNA-199a-3p and miRNA-193b also control the expression of COL2A1 and aggrecan in human OA chondrocytes.20
Conclusions and future directions
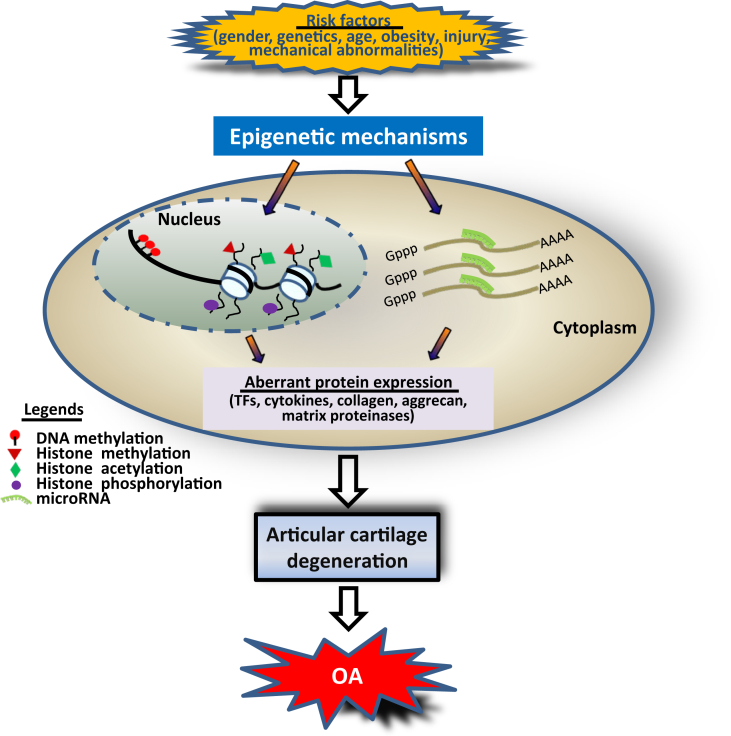
As epigenetics is a molecular link of environmental factors to the development of diseases,64, 65 the advance of epigenetics has greatly enhanced our understanding of the pathogenesis of multifactorial diseases, such as cancer and OA. Accumulative influence of risk factors which trigger epigenetic events such as DNA methylation, histone modifications and miRNAs in chondrocytes, may result in aberrant gene expression of TFs, cytokines, collagen and aggrecan, and matrix proteinases. Abnormal expression of these genes may compromise the balance of anabolic and catabolic activity and disrupt cartilage homeostasis, leading to cartilage degradation, which is the key step of the development of OA (Fig. 1). Moreover, the formation of positive feedback loops among TFs, cytokines and matrix proteinases during the development of OA may partially explain why OA is irreversible once occurs.66
Figure 1.
Possible roles of epigenetic changes in the pathogenesis of OA. Under the accumulative effect of risk factors, chondrocytes undergo epigenetic events including DNA methylation and histone modifications that occur in the nucleus, and miRNAs which function in the cytoplasm. This results in aberrant expression of TFs, cytokines, collagen, aggrecan, and matrix proteinases. Abnormal expression of these factors may disrupt the balance of anabolic and catabolic activity and compromise cartilage homeostasis, leading to articular cartilage degradation and the development of OA.
With the use of new techniques, especially the application of second generation sequencing in the study of epigenetics, the era of epigenetic study in the pathogenesis of OA is coming.67 Global analysis of epigenetic modifications in OA is being undertaken and more detailed epigenetic alterations in OA will be identified.47, 68, 69, 70 The upcoming epigenetic findings may not only broaden our knowledge to appreciate the molecular mechanisms underlying the development of OA, but also promote the development of new drugs for the treatment of OA.71
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by the U.S. National Institute of Health (NIH) Grant R01 AR059088 (to J. Wang), the U.S. Department of Defense Research Grant W81XWH-12-1-0304 (to J. Wang), the Mary A. and Paul R. Harrington Distinguished Professorship Endowment (to J. Wang), and the Asher Orthopedic Research Endowment.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Murphy L., Helmick C.G. The impact of osteoarthritis in the united states: a population-health perspective. Am J Nurs. 2012;112(3 suppl 1):S13–S19. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 2.Carter D.R., Beaupre G.S., Wong M. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004;(427 suppl):S69–S77. doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 3.Chen C., Tambe D.T., Deng L., Yang L. Biomechanical properties and mechanobiology of the articular chondrocyte. Am J Physiol Cell Physiol. 2013;305:C1202–C1208. doi: 10.1152/ajpcell.00242.2013. [DOI] [PubMed] [Google Scholar]
- 4.Blagojevic M., Jinks C., Jeffery A., Jordan K.P. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 7.Vaquero A., Loyola A., Reinberg D. The constantly changing face of chromatin. Sci Aging Knowledge Environ. 2003;2003:Re4. doi: 10.1126/sageke.2003.14.re4. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove M.S., Boeke J.D., Wolberger C. Regulated nucleosome mobility and the histone code. Nat Struct Mol Biol. 2004;11:1037–1043. doi: 10.1038/nsmb851. [DOI] [PubMed] [Google Scholar]
- 9.Egger G., Liang G., Aparicio A., Jones P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 10.Saetrom P., Snove O., Jr., Rossi J.J. Epigenetics and micrornas. Pediatr Res. 2007;61(5 Pt 2):17r–23r. doi: 10.1203/pdr.0b013e318045760e. [DOI] [PubMed] [Google Scholar]
- 11.Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Mattick J.S., Makunin I.V. Non-coding rna. Hum Mol Genet. 2006:R17–R29. doi: 10.1093/hmg/ddl046. 15 Spec No 1. [DOI] [PubMed] [Google Scholar]
- 13.Bartel D.P. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Esteller M. Non-coding rnas in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 15.Stefani G., Slack F.J. Small non-coding rnas in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Gardner B.M., Lu Q. Transcription factor nfat1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J Pathol. 2009;219:163–172. doi: 10.1002/path.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodova M., Lu Q., Li Y. Nfat1 regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. J Bone Miner Res. 2011;26:1974–1986. doi: 10.1002/jbmr.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K.I., Park Y.S., Im G.I. Changes in the epigenetic status of the sox-9 promoter in human osteoarthritic cartilage. J Bone Miner Res. 2013;28:1050–1060. doi: 10.1002/jbmr.1843. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Sanchez A., Dudek K.A., Murphy C.L. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator sox9 by microrna-145 (mirna-145) J Biol Chem. 2012;287:916–924. doi: 10.1074/jbc.M111.302430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ukai T., Sato M., Akutsu H., Umezawa A., Mochida J. Microrna-199a-3p, microrna-193b, and microrna-320c are correlated to aging and regulate human cartilage metabolism. J Orthop Res. 2012;30:1915–1922. doi: 10.1002/jor.22157. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K., Otero M., Imagawa K. Regulated transcription of human matrix metalloproteinase 13 (mmp13) and interleukin-1beta (il1b) genes in chondrocytes depends on methylation of specific proximal promoter cpg sites. J Biol Chem. 2013;288:10061–10072. doi: 10.1074/jbc.M112.421156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto K., Oreffo R.O., Gibson M.B., Goldring M.B., Roach H.I. DNA demethylation at specific cpg sites in the il1b promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 2009;60:3303–3313. doi: 10.1002/art.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santini P., Politi L., Vedova P.D., Scandurra R., Scotto d'Abusco A. The inflammatory circuitry of mir-149 as a pathological mechanism in osteoarthritis. Rheumatol Int. 2014;34:711–716. doi: 10.1007/s00296-013-2754-8. [DOI] [PubMed] [Google Scholar]
- 24.Roach H.I., Yamada N., Cheung K.S. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific cpg sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 25.Cheung K.S., Hashimoto K., Yamada N., Roach H.I. Expression of adamts-4 by chondrocytes in the surface zone of human osteoarthritic cartilage is regulated by epigenetic DNA de-methylation. Rheumatol Int. 2009;29:525–534. doi: 10.1007/s00296-008-0744-z. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q., Zhang X., Dai L. Long noncoding rna related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 2014;66:969–978. doi: 10.1002/art.38309. [DOI] [PubMed] [Google Scholar]
- 27.Young D.A., Lakey R.L., Pennington C.J. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther. 2005;7:R503–R512. doi: 10.1186/ar1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higashiyama R., Miyaki S., Yamashita S. Correlation between mmp-13 and hdac7 expression in human knee osteoarthritis. Mod Rheumatol. 2010;20:11–17. doi: 10.1007/s10165-009-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuda M., Takahashi S., Takahashi Y., Asahara H. Transcriptional co-activators creb-binding protein and p300 regulate chondrocyte-specific gene expression via association with sox9. J Biol Chem. 2003;278:27224–27229. doi: 10.1074/jbc.M303471200. [DOI] [PubMed] [Google Scholar]
- 30.Dvir-Ginzberg M., Gagarina V., Lee E.J., Hall D.J. Regulation of cartilage-specific gene expression in human chondrocytes by sirt1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppenheimer H., Kumar A., Meir H. Set7/9 impacts col2a1 expression through binding and repression of sirt1 histone deacetylation. J Bone Miner Res. 2014;29:348–360. doi: 10.1002/jbmr.2052. [DOI] [PubMed] [Google Scholar]
- 32.Imagawa K., de Andres M.C., Hashimoto K. Association of reduced type ix collagen gene expression in human osteoarthritic chondrocytes with epigenetic silencing by DNA hypermethylation. Arthritis Rheumatol. 2014;66:3040–3051. doi: 10.1002/art.38774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poschl E., Fidler A., Schmidt B. DNA methylation is not likely to be responsible for aggrecan down regulation in aged or osteoarthritic cartilage. Ann Rheum Dis. 2005;64:477–480. doi: 10.1136/ard.2004.022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge M.R., Ranger A.M., Charles de la Brousse F. Hyperproliferation and dysregulation of il-4 expression in nf-atp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 35.Xanthoudakis S., Viola J.P., Shaw K.T. An enhanced immune response in mice lacking the transcription factor nfat1. Science. 1996;272:892–895. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 36.Lefebvre V., Li P., de Crombrugghe B. A new long form of sox5 (l-sox5), sox6 and sox9 are coexpressed in chondrogenesis and cooperatively activate the type ii collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bi W., Deng J.M., Zhang Z., Behringer R.R., de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 38.Henry S.P., Liang S., Akdemir K.C., de Crombrugghe B. The postnatal role of sox9 in cartilage. J Bone Miner Res. 2012;27:2511–2525. doi: 10.1002/jbmr.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J.S., Im G.I. Sox trio decrease in the articular cartilage with the advancement of osteoarthritis. Connect Tissue Res. 2011;52:496–502. doi: 10.3109/03008207.2011.585409. [DOI] [PubMed] [Google Scholar]
- 40.Kypriotou M., Fossard-Demoor M., Chadjichristos C. Sox9 exerts a bifunctional effect on type ii collagen gene (col2a1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol. 2003;22:119–129. doi: 10.1089/104454903321515922. [DOI] [PubMed] [Google Scholar]
- 41.Cucchiarini M., Thurn T., Weimer A. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor sox9. Arthritis Rheum. 2007;56:158–167. doi: 10.1002/art.22299. [DOI] [PubMed] [Google Scholar]
- 42.Feldmann M., Brennan F.M., Maini R.N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 43.Goldring M.B., Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldring M.B. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 45.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 46.Akhtar N., Rasheed Z., Ramamurthy S. Microrna-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–1371. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhtar N., Haqqi T.M. Level of il-1-induced epigenetic modifications differ in chondrocytes from different histological zones of human cartilage [abstract] Arthritis Rheum. 2012;64(suppl 10):29. [Google Scholar]
- 48.Sullivan K.E., Reddy A.B., Dietzmann K. Epigenetic regulation of tumor necrosis factor alpha. Mol Cell Biol. 2007;27:5147–5160. doi: 10.1128/MCB.02429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang K., Wu L.D. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36:1149–1160. doi: 10.1177/147323000803600601. [DOI] [PubMed] [Google Scholar]
- 50.Burrage P.S., Mix K.S., Brinckerhoff C.E. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 51.Tortorella M.D., Malfait A.M., Deccico C., Arner E. The role of adam-ts4 (aggrecanase-1) and adam-ts5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 52.Glasson S.S., Askew R., Sheppard B. Deletion of active adamts5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 53.Stanton H., Rogerson F.M., East C.J. Adamts5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 54.Rogerson F.M., Stanton H., East C.J. Evidence of a novel aggrecan-degrading activity in cartilage: studies of mice deficient in both adamts-4 and adamts-5. Arthritis Rheum. 2008;58:1664–1673. doi: 10.1002/art.23458. [DOI] [PubMed] [Google Scholar]
- 55.Little C.B., Barai A., Burkhardt D. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neuhold L.A., Killar L., Zhao W. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (mmp-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kempson G.E., Muir H., Pollard C., Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta. 1973;297:456–472. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- 58.Rizkalla G., Reiner A., Bogoch E., Poole A.R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992;90:2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan D., Cole W.G., Chow C.W., Mundlos S., Bateman J.F. A col2a1 mutation in achondrogenesis type ii results in the replacement of type ii collagen by type i and iii collagens in cartilage. J Biol Chem. 1995;270:1747–1753. [PubMed] [Google Scholar]
- 60.Fassler R., Schnegelsberg P.N., Dausman J. Mice lacking alpha 1 (ix) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci U S A. 1994;91:5070–5074. doi: 10.1073/pnas.91.11.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saamanen A.K., Salminen H.J., Dean P.B. Osteoarthritis-like lesions in transgenic mice harboring a small deletion mutation in type ii collagen gene. Osteoarthritis Cartilage. 2000;8:248–257. doi: 10.1053/joca.2000.0298. [DOI] [PubMed] [Google Scholar]
- 62.Hollander A.P., Pidoux I., Reiner A. Damage to type ii collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez M.P., Young M.F., Sobel M.E. Methylation of type ii and type i collagen genes in differentiated and dedifferentiated chondrocytes. J Biol Chem. 1985;260:2374–2378. [PubMed] [Google Scholar]
- 64.Ling C., Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718–2725. doi: 10.2337/db09-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skinner M.K., Manikkam M., Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai Y., Bai X., Zhao Y. Adamts-7 forms a positive feedback loop with tnf-alpha in the pathogenesis of osteoarthritis. Ann Rheum Dis. 2014;73:1575–1584. doi: 10.1136/annrheumdis-2013-203561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanco F.J., Rego-Perez I. Editorial: is it time for epigenetics in osteoarthritis? Arthritis Rheumatol. 2014;66:2324–2327. doi: 10.1002/art.38710. [DOI] [PubMed] [Google Scholar]
- 68.Moazedi-Fuerst F.C., Hofner M., Gruber G. Epigenetic differences in human cartilage between mild and severe oa. J Orthop Res. 2014;32:1636–1645. doi: 10.1002/jor.22722. [DOI] [PubMed] [Google Scholar]
- 69.Jeffries M.A., Donica M., Baker L.W. Genome-wide DNA methylation study identifies significant epigenomic changes in osteoarthritic cartilage. Arthritis Rheumatol. 2014;66:2804–2815. doi: 10.1002/art.38762. [DOI] [PubMed] [Google Scholar]
- 70.Rushton M.D., Reynard L.N., Barter M.J. Characterization of the cartilage DNA methylome in knee and hip osteoarthritis. Arthritis Rheumatol. 2014;66:2450–2460. doi: 10.1002/art.38713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roach H.I., Aigner T. DNA methylation in osteoarthritic chondrocytes: a new molecular target. Osteoarthritis Cartilage. 2007;15:128–137. doi: 10.1016/j.joca.2006.07.002. [DOI] [PubMed] [Google Scholar]