Abstract
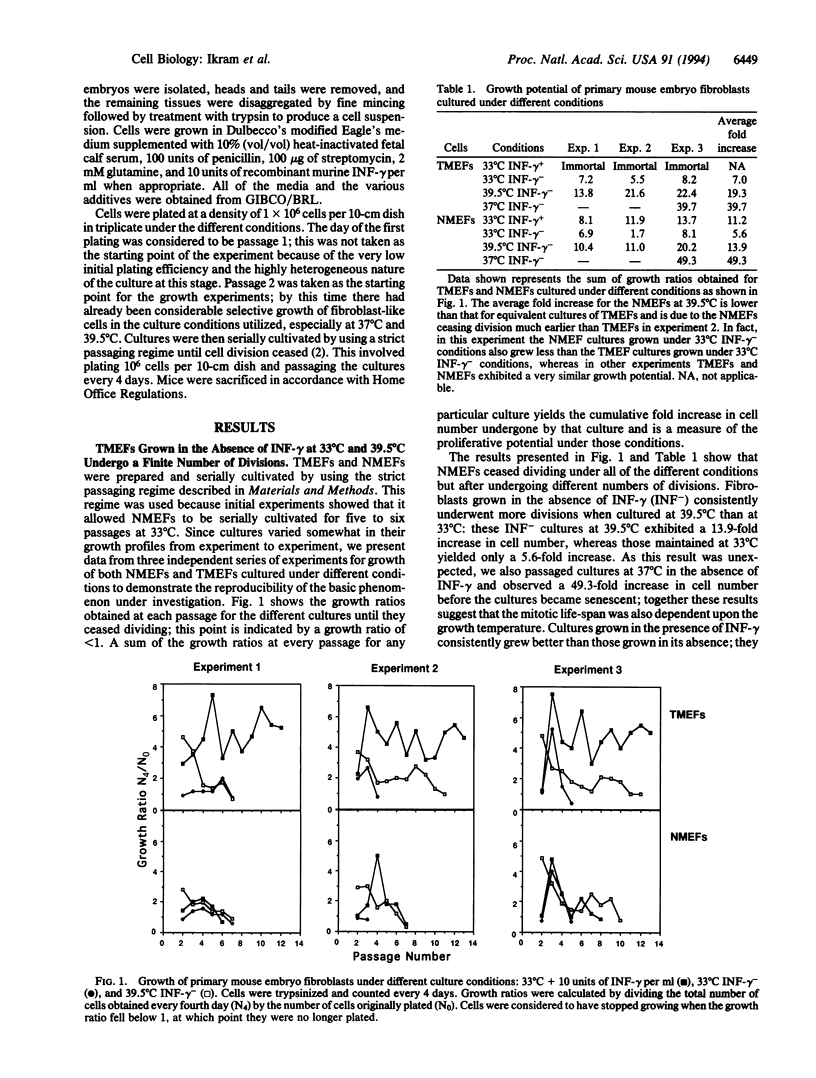
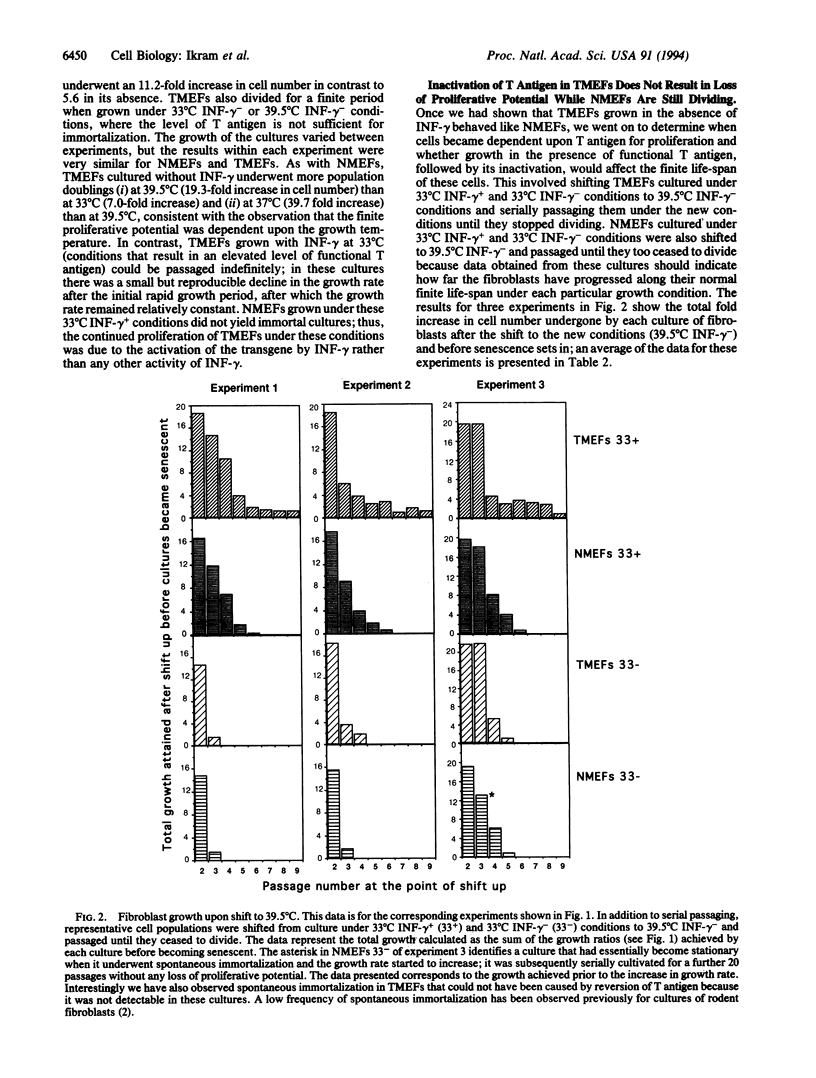
Normal mammalian fibroblasts cultured in vitro undergo a limited number of divisions before entering a senescent phase in which they can be maintained for long periods but cannot be induced to divide. In rodent fibroblasts senescence can be prevented by expression of simian virus 40 large tumor antigen (T antigen). Cells expressing T antigen can proliferate indefinitely; however, such cells are absolutely dependent upon continued expression of T antigen for maintenance of growth; inactivation of T antigen results in a rapid and irreversible entry into a postmitotic state. To determine when, after the initial expression of T antigen, fibroblasts become dependent upon it for continued growth, we serially cultivated embryonic fibroblasts prepared from H-2Kb-tsA58 transgenic mice. We show that these fibroblasts become dependent upon T antigen for maintenance of proliferation only when their normal mitotic life-span has elapsed and that the biological clock that limits the mitotic potential continues to function normally, even in cells expressing this immortalizing gene. Our results suggest that random accumulation of cellular damage is unlikely to be the factor that limits fibroblast division but support the hypothesis that senescence is regulated via a genetic program.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayreuther K., Rodemann H. P., Hommel R., Dittmann K., Albiez M., Francz P. I. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögler O., Noble M. Measurement of time in oligodendrocyte-type-2 astrocyte (O-2A) progenitors is a cellular process distinct from differentiation or division. Dev Biol. 1994 Apr;162(2):525–538. doi: 10.1006/dbio.1994.1106. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Watine B., Israël A., Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today. 1990 Aug;11(8):286–292. doi: 10.1016/0167-5699(90)90114-o. [DOI] [PubMed] [Google Scholar]
- Dell'Orco R. T., Mertens J. G., Kruse P. F., Jr Doubling potential, calendar time, and senescence of human diploid cells in culture. Exp Cell Res. 1973 Mar 15;77(1):356–360. doi: 10.1016/0014-4827(73)90588-0. [DOI] [PubMed] [Google Scholar]
- Faragher R. G., Kill I. R., Hunter J. A., Pope F. M., Tannock C., Shall S. The gene responsible for Werner syndrome may be a cell division "counting" gene. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12030–12034. doi: 10.1073/pnas.90.24.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano T., Foster D. N. Identification of a highly abundant cDNA isolated from senescent WI-38 cells. Exp Cell Res. 1989 Dec;185(2):399–406. doi: 10.1016/0014-4827(89)90310-8. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Singal D. P. Senescence of cultured human fibroblasts: mitotic versus metabolic time. Exp Cell Res. 1974 Oct;88(2):359–364. doi: 10.1016/0014-4827(74)90252-3. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Goldstein S. Cultured human fibroblasts: distribution of cell generations and a critical limit. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):509–516. doi: 10.1002/jcp.1040970326. [DOI] [PubMed] [Google Scholar]
- Harvey D. M., Levine A. J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991 Dec;5(12B):2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Israel A., Kimura A., Fournier A., Fellous M., Kourilsky P. Interferon response sequence potentiates activity of an enhancer in the promoter region of a mouse H-2 gene. Nature. 1986 Aug 21;322(6081):743–746. doi: 10.1038/322743a0. [DOI] [PubMed] [Google Scholar]
- Jat P. S., Noble M. D., Ataliotis P., Tanaka Y., Yannoutsos N., Larsen L., Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol. 1989 Apr;9(4):1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J Virol. 1986 Sep;59(3):746–750. doi: 10.1128/jvi.59.3.746-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J. A., Voulalas P., Roeder D., Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990 Sep 28;249(4976):1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Murano S., Thweatt R., Shmookler Reis R. J., Jones R. A., Moerman E. J., Goldstein S. Diverse gene sequences are overexpressed in werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol. 1991 Aug;11(8):3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuell M. J., Stewart D. A., Walker L., Friedman V., Wood C. M., Owens G. A., Smith J. R., Schneider E. L., Dell' Orco R., Lumpkin C. K. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol Cell Biol. 1991 Mar;11(3):1372–1381. doi: 10.1128/mcb.11.3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E. Ageing of clones of mammalian cells. Nature. 1973 Jun 22;243(5408):441–445. doi: 10.1038/243441a0. [DOI] [PubMed] [Google Scholar]
- Petit C. A., Gardes M., Feunteun J. Immortalization of rodent embryo fibroblasts by SV40 is maintained by the A gene. Virology. 1983 May;127(1):74–82. doi: 10.1016/0042-6822(83)90372-0. [DOI] [PubMed] [Google Scholar]
- Sherwood S. W., Rush D., Ellsworth J. L., Schimke R. T. Defining cellular senescence in IMR-90 cells: a flow cytometric analysis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9086–9090. doi: 10.1073/pnas.85.23.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Drullinger L. F., Robetorye R. S., Pereira-Smith O. M., Smith J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilard L. ON THE NATURE OF THE AGING PROCESS. Proc Natl Acad Sci U S A. 1959 Jan;45(1):30–45. doi: 10.1073/pnas.45.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. Tumor suppressor genes. Neuron. 1993 Aug;11(2):191–196. doi: 10.1016/0896-6273(93)90177-s. [DOI] [PubMed] [Google Scholar]



