Abstract
T cells genetically modified to express a CD19-specific chimeric antigen receptor (CAR) for the investigational treatment of B-cell malignancies comprise a heterogeneous population, and their ability to persist and participate in serial killing of tumor cells is a predictor of therapeutic success. We implemented Timelapse Imaging Microscopy In Nanowell Grids (TIMING) to provide direct evidence that CD4+CAR+ T cells (CAR4 cells) can engage in multi-killing via simultaneous conjugation to multiple tumor cells. Comparisons of the CAR4 cells and CD8+CAR+ T cells (CAR8 cells) demonstrate that while CAR4 cells can participate in killing and multi-killing, they do so at slower rates, likely due to the lower Granzyme B content. Significantly, in both sets of T cells, a minor sub-population of individual T cells identified by their high motility, demonstrated efficient killing of single tumor cells. By comparing both the multi-killer and single killer CAR+ T cells it appears that the propensity and kinetics of T-cell apoptosis was modulated by the number of functional conjugations. T cells underwent rapid apoptosis, and at higher frequencies, when conjugated to single tumor cells in isolation and this effect was more pronounced on CAR8 cells. Our results suggest that the ability of CAR+ T cells to participate in multi-killing should be evaluated in the context of their ability to resist activation induced cell death (AICD). We anticipate that TIMING may be utilized to rapidly determine the potency of T-cell populations and may facilitate the design and manufacture of next-generation CAR+ T cells with improved efficacy.
INTRODUCTION
Chimeric antigen receptors (CARs, glossary of abbreviations in supplementary information) are hybrid molecules that typically combine the specificity and affinity of single-chain antibodies with selected intracellular signaling domains of the T-cell receptor (TCR) complex1-3. When expressed on genetically modified T cells, CARs redirect specificity independent of human leukocyte antigen (HLA) to recognize tumor-associated antigens (TAAs). Second and third generation CARs include the endodomains for co-stimulatory molecules and can thus directly endow the different signals needed for T-cell activation upon binding TAA4. Initial data from clinical trials at multiple centers reporting the adoptive transfer of T cells genetically modified to express a CD19-specific CAR for the treatment of B-cell malignancies are encouraging, with patients benefiting from complete remissions5-7. These clinical results have accelerated the clinical translation of T cells bearing CARs targeting TAAs other than CD19 for the treatment of hematologic malignancies as well as solid tumors8-10. As a group, these clinical trials differ in the design and specificity of the CARs, the ex vivo approach used to manufacture the T cells, the in vivo regimen used to pre-treat the recipient, the tumor burden and type, and the T-cell dosing scheme. Thus, drawing conclusions regarding the relative anti-tumor effects between the populations of bioengineered CAR+ T cells is not readily feasible1. One of the hallmarks of a therapeutically successful infusion is the presence of CAR+ T cells that can persist to execute multiple tumor cells within the tumor microenvironment11.
In spite of the recent success of adoptive immunotherapy, the mechanistic basis for the potency of a given T-cell product has not been well defined. The majority of adoptive studies have focused on infusing CD8+ T-cell populations because of their ability to directly recognize and lyse tumor cells, thus mediating antitumor immunity12. In the absence of CD4+ T-cell help however, some infused CD8+ T cells can become functionally unresponsive and undergo apoptosis13. Indeed, adoptive cell therapy (ACT) protocols that incorporate CD4+ T cells may mediate superior responses, and preclinical and clinical data have established the importance of CD4+ T-cell help during immunotherapy14,15. More recently however, adoptive transfer of CD4+ T-cell populations has shown that these cells can mediate regression of established melanoma, and that these cells can differentiate into cytolytic effectors16-18. Despite these advances direct comparisons of the potency and kinetics of interactions between donor-derived populations of CD4+ T cells and tumor cells at single-cell resolution, and the comparison to CD8+ T cells is lacking.
Although two-photon microscopy studies are well suited for understanding the mechanistic basis of T-cell tumor cell interactions in vivo, direct observation of killing and motility is restricted to tens of events that may lead to sampling bias. Additionally, these studies are limited in throughput and cannot be used to routinely determine the interactions between cellular infusions and tumor cells. In vitro dynamic imaging19-24 systems are well-suited for studying the longitudinal interactions between cells at single-cell resolution, in a defined environment. Here, we have employed Timelapse Imaging Microscopy In Nanowell Grids (TIMING) to analyze the longitudinal interactions between individual CD19-specific T cells (effectors, E) expressing a second generation CAR with one or more CD19+ tumor cells (target(s), T). To the best of our knowledge, we demonstrate for the first time that CD4+CAR+ T cells (CAR4 cells) can directly engage in multi-killing via simultaneous conjugation to multiple tumor cells. The major differences between CAR4 and CD8+ CAR+ T cells (CAR8 cells), at the single-cell, in mediating tumor-cell lysis in vitro, was the kinetics of killing, and this was attributed to the differences in their intracellular Granzyme B (GzB) content. Surprisingly, in both sets of T cells, a minor sub-population of individual T cells identified by their high motility, demonstrated efficient killing of single tumor cells. By comparing both the multi-killer and single killer CAR+ T cells it appears that the propensity and kinetics of T-cell apoptosis was modulated by the number of functional conjugations. Our results demonstrate that the ability of CAR+ T cells to participate in multi-killing should be evaluated in the context of their ability to resist AICD.
METHODS
Human Subjects Statement
All work outlined in this report was performed according to protocols approved by the Institutional Review Boards at the University of Houston and the University of Texas M.D. Anderson Cancer Center.
Cell Lines and antibodies
All antibodies were purchased from Biolegend (San Diego, CA). Human pre-B cell line NALM-6 (ATCC), Daudi-β2m (ATCC), T-cell lymphoma EL-4 (ATCC) and modified CD19+EL-4 cells were cultured as described previously25,26. The cell lines were routinely tested to ensure that they were free of mycoplasma contamination and flow-cytometry was utilized to confirm the expression of CD19.
Genetic modification and propagation of cells
PBMC from healthy volunteers were electroporated using Nucleofector II (Amaxa/Lonza) with DNA plasmids encoding for second generation CAR (designated CD19RCD28) and SB11 transposase and co-cultured with γ-irradiated K562 aAPC (clone 4) for 28 days along with cytokines (IL-2 and IL-21) in a 7-day stimulation cycle as described previously25. For single cell analysis, frozen CAR+ T cells were revived and re-stimulated with irradiated K562 aAPC before using them in experiments.
Flow cytometry
Cells were stained for cell surface markers (CAR, CD4, CD8, CD3), fixed and permeabilized (Cytofix/Cytoperm, BD Biosciences) for 20 min at 4°C. Cells were subsequently stained for intracellular granzyme B in perm/wash buffer at 4°C for 30 min, acquired on a FACS Calibur, and analyzed using FCS Express/FlowJo as previously described25. Statistical analyses for determining GzB expression were performed within R.
End-point cytotoxicity assay
Nanowell array fabrication and the corresponding cytotoxicity assay to interrogate effector-target interaction at single-cell level were performed as described previously21. Briefly, CAR+ T cells labeled with 1 µM of red fluorescent dye, PKH26 (Sigma) and target cells labeled with 1 µM of green fluorescent dye PKH67 were co-loaded onto nanowell arrays at a concentration of 106 cells/mL. Images were acquired on a Carl Zeiss Axio Observer fitted with a Hamamatsu EM-CCD camera using a 10× 0.3 NA objective. Automated image acquisition of the entire chip was performed at 0 and 6 hour and apoptosis was identified by staining with AnnexinV conjugated to Alexa-647 (Life Technologies, Carlsbad, CA).
TIMING assays
Nanowell grids were fixed in position on a 60 mm petridish. The cells were labeled and loaded exactly as described for the end-point assay and imaged on a Zeiss Axio Observer using a 20× 0.45 NA objective. Images were acquired for 12-16 hours at intervals of 7-10 minutes.
Statistical analysis
The test used to determine p-values are listed in the legend of each figure.
Flow Cytometry based cytotoxicity assay
CAR4 cells (1×106 cells) were incubated with CD19+ target cells (0.2×106 cells; Daudiβ2m, NALM-6, CD19EL-4) at E:T ratio of 5:1 in the presence or absence of 5mM EGTA in 24-well plates in 5% CO2 at 37°C for 6 hours. Following incubation cells were stained for CD3 (T cells) and CD19 (tumor targets), acquired on a FACS Calibur (BD Biosciences) and analyzed using FCS Express version 3.00.007(Thornhill, Canada).
SI Methods contains a description of the image segmentation and tracking algorithms.
RESULTS
Production and phenotype of CAR+ T cells
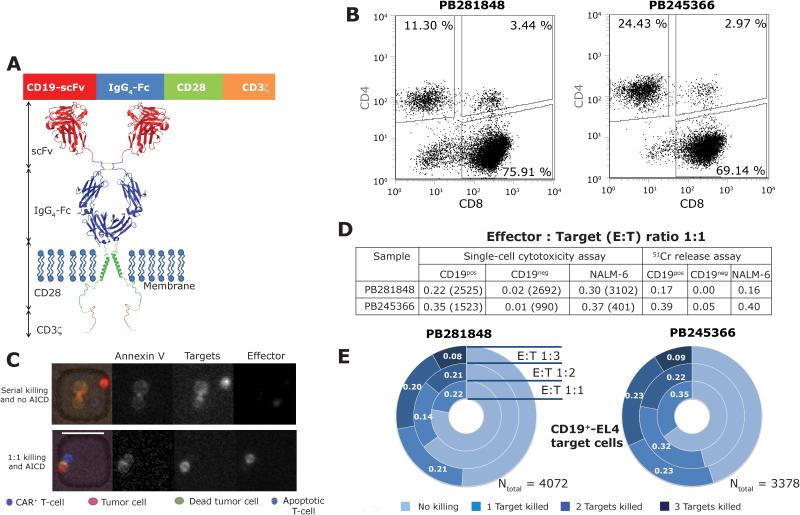
Genetically modified and propagated T cells were generated from the peripheral blood mononuclear cells (PBMC) of healthy volunteer donors derived using the Sleeping Beauty (SB) system27 to enforce expression of a second generation CD19-specific CAR (designated CD19RCD28) that activates T cells via a chimeric CD3 and CD28 endodomain (Figures 1A). Subsequent to expansion, CAR+ T cells from two separate donors contained predominantly CD8+ T cells (Figure 1B). The approach to producing the CAR+ T cells mirrors our manufacture in compliance with current good manufacturing practice for human application (Figures S1 and S2).
Figure 1. High-throughput single-cell analysis of CAR+ T-cell cytolytic functionality in nanowell grids.
(A) Schematic of second-generation CD19-specific CAR (CD19RCD28) that signals through chimeric CD28/CD3-ζ. (B) Phenotypic characterization of the CAR+ T cells from two separate donors. The total CD3+CAR+ population was gated to reveal the frequencies of CD4+ and CD8+ CAR+ T-cell populations. (C) Representative composite micrographs illustrating the ability of single CAR+ T cells to kill, and to undergo apoptosis, when incubated with tumor cells confined within nanowells. Scale bar 50 µm. (D) Comparison of the cytolytic responses measured by the single-cell assay and population-level 51Cr release assay, at an E:T ratio of 1:1. The numbers in parentheses for the single-cell assay report the total number of events observed. (E) Donut plots summarizing the frequency of killing outcomes of the interaction between CAR+ T cells, derived from these two donors, and CD19+EL4 target cells. Representative micrographs illustrating each of these interactions are shown in Figure S5.
The cytotoxic potential, specificity and multi-killing ability of individual CAR+ T cells
Donor-derived CAR+ T-cell populations were evaluated for their ability to lyse CD19+EL4 target cells, by co-culture within nanowell grids (Figures 1C and S3). At an E:T of 1:1, averaged across both donors, 29% of single CAR+ T cells induced apoptosis of (number of events, Ntotal = 4,048) CD19+EL4 cells within six hours, whereas they induced apoptosis of just 1% (Ntotal = 3,682) of CD19−EL4 cells in the same time frame. The >29-fold increase of lysis of CD19+ versus CD19− targets confirms TAA-specific lysis (Figure 1D, p-value <0.0001, Fisher’s 2x2 test). In parallel, a conventional 4-hour 51Chromium release assay (CRA) was performed at the same E:T ratio (1:1) and reported a similar overall magnitude of target cells killing (mean 14-fold increase of lysis of CD19+ versus CD19−EL4 cells), albeit without single-cell resolution (Figure 1D). The ability to redirect specificity to lyse human CD19+ tumor cells was confirmed using the pre-B cell line NALM-6 (Figure S4). When averaged across both donors, within six hours of observation, individual CAR+ T cells induced apoptosis in 34% (Ntotal = 3,503) of NALM-6 target cells at an E:T ratio of 1:1. Across all of the samples tested, single cell assay demonstrated a linear correlation to the CRA (Figure 1D, r2 = 0.84, p-value = 0.01). The ability of individual T cells to eliminate more than one target cell was quantified by analyzing nanowells containing multiple targets (Figure S5). Averaged across both donors, at an E:T ratio of 1:2, within six hours, 21% (Ntotal = 2,294) of single CAR+ T cells killed exactly one CD19+EL4 target-cell whereas 23% killed both targets (Figure 1E). During this same timeframe, at an E:T ratio of 1:3, 22% (Ntotal = 1,108) of single CAR+ T cells killed exactly one target, 22 % killed exactly two targets, and 9% killed all three targets (Figure 1E). Thus, within a defined observation window, the likelihood that an individual CAR+ T cell killed more than one tumor cell improved as the number of targets within the nanowell increased but this might simply reflect higher frequency of interactions at higher cell densities (Figure S6). These findings were also observed when substituting NALM-6 as target cells, albeit with diminished frequency of multi-killing after 6 hours of co-culture (Figure S7). In aggregate, these data demonstrate that the responses measured by the single-cell assay are consistent with the results of CRA, and that multi-killer CAR+ T cells (ability to lyse at least two targets) comprised 20% (Ntotal = 3,402) of the CAR+ T-cell population.
Motile CD8+ cytotoxic T cells are efficient killers with decreased potential for activation induced cell death (AICD)
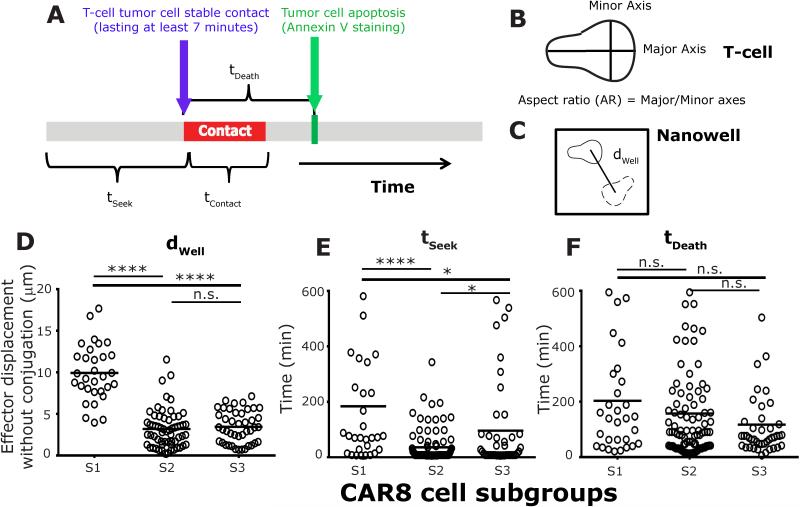
In order to gain an improved mechanistic understanding on the interaction between individual CAR+ T cells and NALM-6 tumor cells, we developed and implemented TIMING (Figure S8). Six parameters describing T-cell intrinsic behavior motility (dWell) and aspect ratio of polarization (AR), conjugation (contact lasting >7 minutes, tSeek and tContact), and death (tDeath and tAICD) were computed to define each interacting pair of effector and tumor cell (Figure 2A-C). At an E:T of 1:1, 77 % (Ntotal = 268) of single CD8+CAR+ T cells (CAR8 cells) that made at least one conjugate were able to kill the engaged leukemia cell. In order to identify subgroups of T cells that exhibited different behavioral interactions with the tumor cells leading to subsequent killing, the time series data for each of three features, total duration of conjugation, dwell and AR, underwent hierarchical clustering (Figure S9)28. Three T-cell subgroups were described that collectively accounted for 70% of the single-killer CAR8 cells: S1 (14% [7-20%], range), low conjugation and high motility; S2 (49% [32-66%]), high conjugation and low motility; and S3 (21% [19-22 %]), low conjugation and low motility (Figure S9). The high-motility subgroup, S1, comprised predominantly of elongated T cells that had an initial “lag-phase” (tSeek 184±38 minutes, Mean±SEM), but formed stable conjugates (tContact 98±13 minutes) prior to target apoptosis (tDeath 204±35 minutes) (Figures 2D-F and S10). Predominantly, these T cells exhibited a decrease in motility and increased circularization (Figure S11) during tumor-cell conjugation, detached after tumor-cell death, resumed normal migratory function and had only a low frequency of effector cells undergoing AICD (Figure S12, Movie M1). The representative cell in the dominant subgroup, S2, established conjugation quickly (tSeek 36±6 minutes), and displayed sustained conjugation (tContact 145±16 minutes) prior to killing (tDeath 158±18 minutes) (Figures 2E-F). The majority of these T cells did not detach or resume migratory function after tumor-cell lysis, retained a predominantly circular morphology, and continued to remain conjugated >10 hours, even subsequent to the death of the conjugated tumor-cell (Movie M2). Moreover, 88% of S2 effector cells underwent apoptosis within the first ten hours of observation (Figure S12). Finally, T cells in the S3 subgroup were rapid killers (tContact 84±8 minutes and tDeath 118±20 minutes) that arrested after conjugation but failed to resume migration after tumor-cell detachment/killing (Figure 2E-F and Movie M3). Although these S3 effectors detached from tumor-cells after delivering the lethal hit, 53% then underwent apoptosis (Figure S12). Taken together these results demonstrate that at an E:T ratio of 1:1, the dominant subgroup of cells, S2, identified by their lack of motility and early conjugation to tumor cell, underwent AICD. On the contrary, highly motile CAR8 cells, S1, detached efficiently and resumed exploration of the local microenvironment, indicating that the motility of CAR8 cells might help identify efficient killers with decreased propensity for AICD. The observation that the majority of the CAR8 cells (S2 subgroup) maintained extended contact even after the death of the tumor cell is consistent with investigations on HIV-specific CTLs29.
Figure 2. CAR8 cells can be classified into different subgroups based on their the motility and conjugation periods with NALM-6 tumor cell (E:T 1:1).
Schematic depicting the effector parameters used to describe their interaction with single NALM-6 tumor cells: (A) Red bar indicates periods of conjugation, blue arrow indicates timepoint at which conjugation was first observed, and green line indicates time to target death since first conjugation. (B) Aspect ratio of polarization describes the ratio of major and minor axis fitted to an ellipse. (C) dWell represents the average displacement of the centroid of the effector cell between successive seven minute time points. The mean: (D) motility, (E) time to first conjugation, and (F) killing efficiency, of single CAR8 cells in each of three different subgroups. Each circle represents a single cell. P-values for multiple comparisons were computed using parametric one-way ANOVA.
CAR8 cell motility at increased tumor-cell densities facilitates multiplexed killing
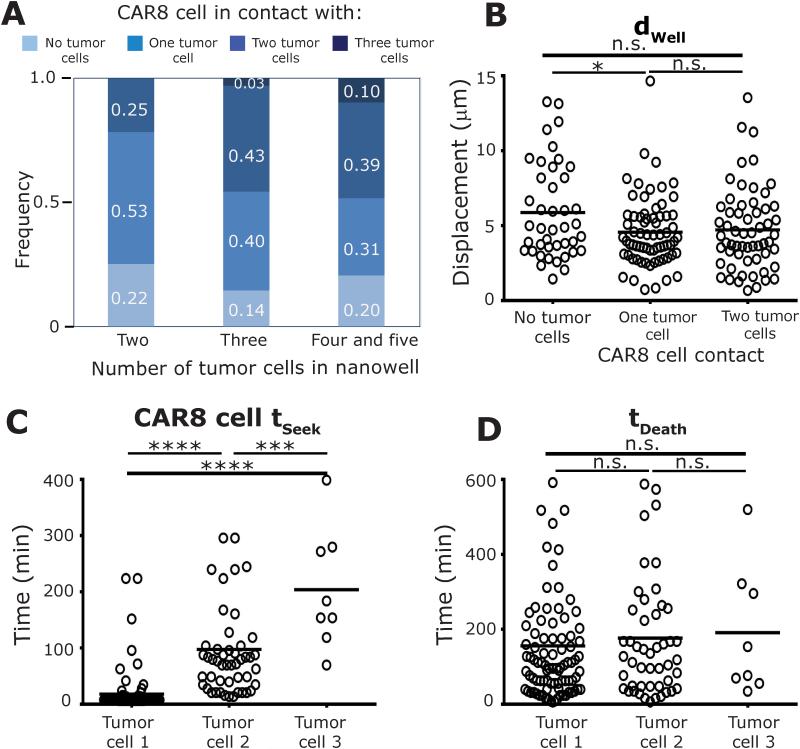
The efficacy of CAR+ T cells to eliminate tumor burden in excess of the number of effectors infused is due to their ability to persist and participate in serial killing11. To facilitate identification of multi-killers, we next profiled the interactions in nanowells containing a single CAR8 cell and 2 to 5 NALM-6 tumor cells (E:T 1:2-5). The frequency of CAR8 cells that were able to simultaneously conjugate to two or more tumor cells increased from 25% to 49% as the number of targets within the nanowell increased, indicating that multiplexed killing might be important (Figure 3A and Movie M4). The frequency of simultaneous tumor conjugates that result in tumor cell deaths (46% [43-50%]) was not very different from true serial killers that attach, kill, detach and attach to a different tumor cell (49% [44-53%]), suggesting that CAR8 cells are capable of eliciting either mode of killing, likely dependent on tumor cell density. Individual multi-killer CAR8 cells (Ntotal = 70) demonstrated only a small decrease in motility when conjugated to one tumor cell but showed no significant change in motility upon conjugation to multiple tumor cells (dWell(unconjugated): 5.9±0.5 µm vs dWell (single target): 4.6±0.3 µm vs dWell (two targets): 4.7±0.3 µm) (Figure 3B). The only difference for multi-killers when contacting the different tumor cells was in their time to establish conjugates (tSeek Target1: 18±4 minutes vs Target2: 98±13 minutes, Figure 3C). Both, duration of conjugation (tContactTarget1: 101±9 minutes vs Target2: 113±15 minutes) and killing efficiency (tDeathTarget1: 156± 17 minutes vs Target2: 177±24 minutes) were no different (Figure 3D and S13). In addition to contact duration, the number of CAR8 cell tumor cell conjugations that lead to killing during encounter with the first tumor cells (61% both donors) was also not significantly different from the number of conjugations that resulted in target cell killing during encounter with the second tumor cell (74% [70-79 %]). These TIMING data suggest that the efficiency to kill a second tumor cell is largely unaffected by the hit on a first target (p-value >0.99). Furthermore, in comparison to single killer CAR8 cells, multi-killer CAR8 cells displayed greater motility when conjugated to the tumor cell despite the increased crowding because of higher tumor cell density, (Figure S14).
Figure 3. Multi-killer CAR8 cells engage in simultaneous conjugations leading to multiplexed killing (E:T 1:2-5).
(A) Distribution of the number of simultaneous conjugations of individual CAR8 cells when incubated with increasing number of NALM-6 tumor cells. The mean: (B) motility, (C) time to first conjugation, and (D) killing efficiency, of individual multi-killer CAR8 cells. P-values for multiple comparisons were computed using parametric one-way ANOVA.
Motility can identify a subgroup of CAR4 cells with enhanced cytotoxic efficiency
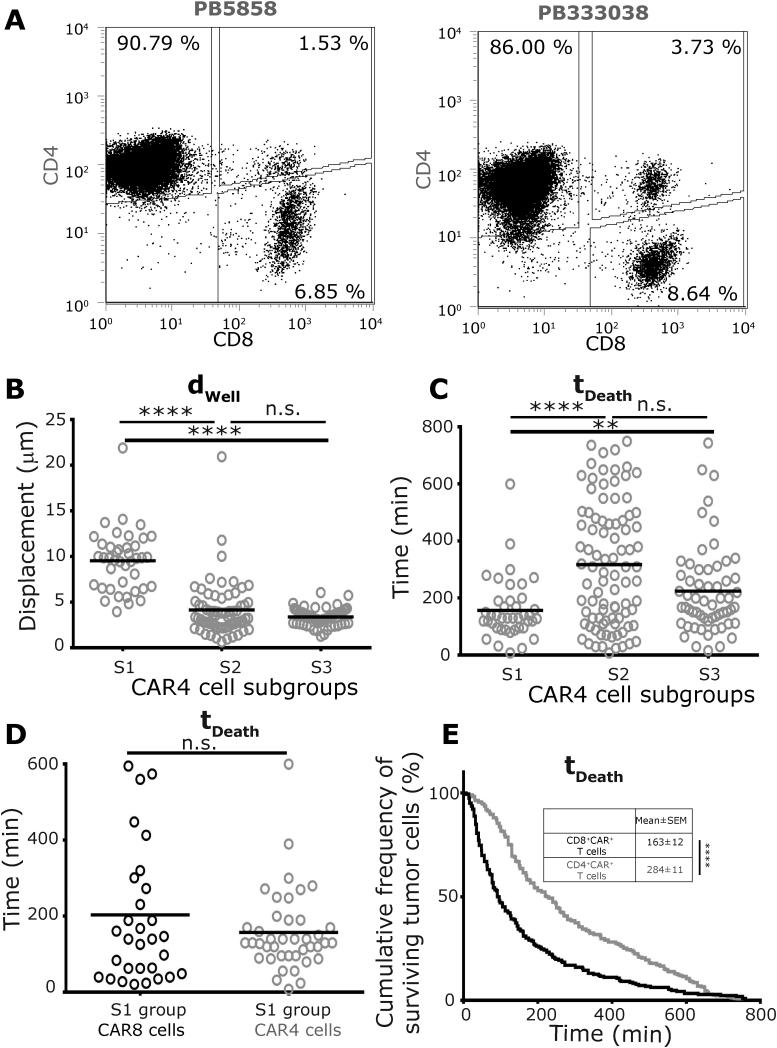
We have previously reported that the culture of CAR+ T cells in the presence of IL-2 and IL-21 on aAPC can lead to outgrowth of CAR4 cells with cytotoxic potential25. In order to facilitate comparisons to CAR8 cells, and to demonstrate that CAR4 can directly participate in killing and multi-killing (Movie M5), the interaction of individual CAR4 cells from two donor-derived populations (Figure 4A), with NALM-6 tumor cells were profiled using TIMING. At an E:T ratio of 1:1, 55% (Ntotal = 549) of single CAR4 cells that conjugated to a NALM-6 cell subsequently killed the tumor cell. As with the CAR8 cells, the interaction behavior of CAR4 cells with the NALM-6 cells could be classified into three subgroups, S1-S3 (Figure S15). CAR4 cells in the enhanced motility subgroup, S1 (11% both donors), displayed significantly faster kinetics of tumor cell death (tDeath 157±17 minutes) compared to the dominant S2 (34% [31-36 %]) subgroup (tDeath 318±23 minutes, Figure 4B-D). This increased kinetic efficiency was consistent with the decreased conjugation time required by the S1 subgroup of cells (tContact 122±11 minutes) in comparison to the S2 subgroup (tContact 300±21 minutes) (Figure S16). These results suggest that similar to CAR8 cells, the motility of the CAR4 cells may help identify the most efficient killers.
Figure 4. Subpopulation of CAR4 cells, identified based on their motility, can engage in efficient killing (E:T 1:1).
(A) Phenotypic characterization of the CAR+ T cells from two separate donors that comprise of predominantly CD4+CAR+ T cells. The mean: (B) motility, and (C) killing efficiency, of single CAR4 cells in each of three different subgroups. (D) Comparison of the means of the killing efficiencies between single CAR8 and CAR4 cells within the S1 subgroups. Each circle represents a single cell in panels B-D; CAR4 cells are represented using grey circles and CAR8 cells are represented using black circles. (E) Comparative Kaplan –Meier estimators depicting the differences in killing efficiencies of the entire population of CAR4 cells and CAR8 cells. P-values for multiple comparisons (B/C) were computed using a parametric one-way ANOVA, and dual comparisons (D/E) computed using unpaired two-tailed t-test.
Both single-killer and multi-killer CAR4 cells required longer conjugation and demonstrated delayed kinetics of killing in comparison to CAR8 cells
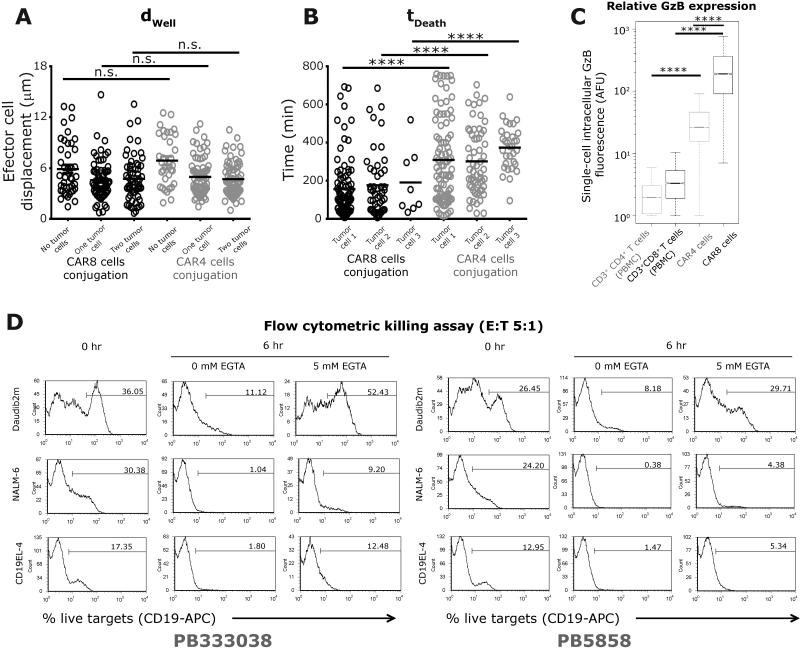
At the E:T ratio of 1:1, comparisons of the killing efficiency of CAR4 cells (tDeath 284±11 minutes) and CAR8 cells (163±12 minutes) demonstrated that individual CAR4 cells on average required two extra hours to induce tumor cell death (Figure 4E). Consistent with the observation that the S2 subgroup is the dominant population of CAR+ T cells, CAR4 cells in the S2 subgroup (tDeath 318±23 minutes) demonstrated delayed kinetics of killing in comparison to CAR8 cells within the S2 subgroup (tDeath 158±18 minutes) (Figure S17). As mentioned above, since the motility of CAR4 cells could be used to identify the most efficient killers (Figure 4C), comparisons of the kinetic efficiency of CAR4 cells in the S1 subgroup (tDeath 157±17 minutes) with CAR8 cells in the S1 subgroup (tDeath 204±34 minutes) demonstrated no significant differences. This further supports the notion that motility might be a useful parameter in identifying efficient cytolytic CAR+ T cells. Comparisons of the single-cell behavioral interactions of multi-killer CAR4 cells (Ntotal = 78) with the CAR8 cells demonstrated that most features were conserved across cells of both phenotypes. First, the unconjugated motility of CAR4 cells (dwell 6.9±0.5 µm) was no different than CAR8 cells (dwell 5.9±0.5 µm, Figure 5A). Second, like CAR8 cells, CAR4 cells demonstrated a matched decrease in motility (Figure 5A) and increased circularization when conjugated to one or more tumor cells (Figure S18). Third, the preferred contact mode of the multi-killer CAR4 cells was also simultaneous conjugations to multiple tumor cells (Figure S19 and Movie M5). Fourth, simultaneous conjugates that result in killing accounted for 61% [60-63%] of multi killing events, indicating that this is an important mode of killing intrinsic to T cells and not just CD8+ T cells. Fifth, comparisons of tDeath for the different tumor cells killed by individual multi-killer CAR4 cells demonstrated no differences (Figure 5B). Lastly, the number of CAR4 cell tumor cell conjugations that lead to killing during the first tumor cell encounter (60% [58-61 %]) is not significantly different from the number of contacts that leads to killing when encountering the second tumor cell (60% [57-63 %]), suggesting that the killing efficiency is unchanged. Consistent with the observations at an E:T of 1:1, multi-killer CAR4 cells required extended conjugation (tContact 214±18 minutes) and demonstrated slower kinetics prior to killing the first tumor cell (tDeath 310±23 minutes) in comparison to CAR8 cells (Figure 5B). In aggregate these results demonstrate that the major difference in CAR4 cells and CAR8 cells participating in either single killing or multi-killing is the kinetics of tumor cell death.
Figure 5. Multi-killer CAR4 cells demonstrated delayed kinetics of killing in comparison to CAR8 cells (E:T 1:2-5).
Comparisons between the mean: (A) motility, and (B) killing efficiency, of single multi-killer CAR8 cells and CAR4 cells. Each circle represents a single cell; CAR4 cells are represented using grey circles and CAR8 cells are represented using black circles. (C) Box and whisker plots (extremities indicate 99% confidence intervals) displaying intracellular expression of Granzyme B identified by immunofluorescent staining and flow-cytometry. CAR4 cells (from donors PB5858 and PB333038) and CAR8 cells (from donors PB243566 and PB281848) were profiled using mAb against CD4/CD8/CAR and GzB. P-values were computed using parametric one-way ANOVA for multiple comparisons or t-tests for dual comparisons. (D) Flow cytometric killing assay (E:T = 5:1) of CAR4 cells incubated with three separate target cell lines (Daudi-β2m, NALM-6 and CD19+EL4) in the absence or presence of 5mM EGTA blockade.
Intracellular GzB content can explain differences in killing efficiency
To test the hypothesis that the varying efficiencies both between cells of the same population and in comparing CAR4 cells with CAR8 cells might be due to differences in expression of cytotoxic enzymes, we employed intracellular staining at the single-cell level using flow cytometry to identify the expression GzB within these cells. To establish baseline controls, the intracellular GzB content of CD3+CD4+ cells (2.36±0.01) and CD3+CD8+ cells (3.89±0.04) in PBMC of two separate donors was determined (Figure 5C). Consistent with our previous reports, both CAR4 cells (38.6±0.2) and CAR8 cells (267±2) showed significantly increased expression of GzB, in comparison to the controls (Figure 5C). In agreement with the killing efficiency data (Figure 5B), CAR4 cells expressed lower amounts of GzB in comparison to CAR8 cells, suggesting that the origin of the differing kinetic efficiencies of these cells might be the differences in GzB content (Figure 5C).
In order to quantify the contribution GzB secretion to tumor cell killing at the single cell level, the ability of CAR4 cells to kill tumor cells in the presence of the calcium chelator EGTA was studied using flow cytometry30. EGTA blocks cytotoxic granule exocytosis, and hence should eliminate GzB mediated killing. Not surprisingly, CAR4 cells co-cultured with tumor cells in the presence of 5 mM EGTA, demonstrated a substantial reduction in tumor cell killing across three different cell lines, Daudi-β2m, NALM-6 and CD19+EL4 (Figure 5D). The most striking reduction was seen with Daubi-β2m tumor cells, wherein CAR4 cell mediated killing was completely abolished (Figure 5D).
CAR+ T-cell fate is dependent on tumor-cell density
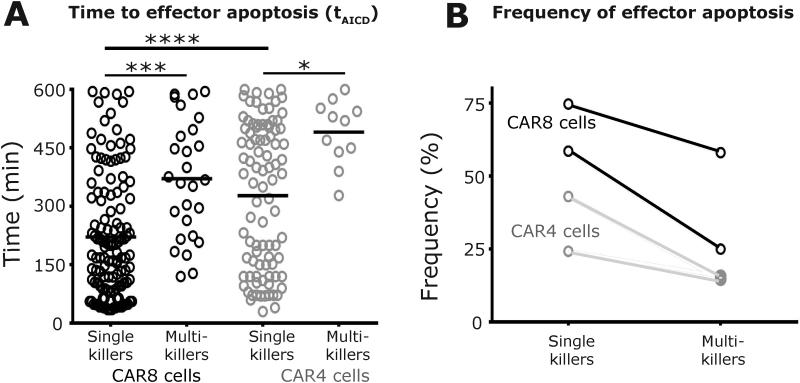
AICD is a mechanism by which T cells undergo programmed apoptosis in response to functional activation31. The frequency and kinetics of individual cytolytic CAR+ T cells to undergo AICD was monitored under the two conditions: at high and low tumor densities. CAR8 cells inducing apoptosis of single targets demonstrated significantly faster kinetics of AICD (tAICD 221±14 minutes) in comparison to the multi-killer CAR8 cells from the same donors (tAICD 371±29 minutes, Figure 6A). This trend of faster AICD kinetics at lower tumor cell density was also observed with CAR4 cells, although with delayed kinetics (Figure 6A). Direct comparisons of the cells of different phenotypes at the same tumor cell density indicated that single-killer CAR8 cells underwent faster AICD (tAICD, 221±14 minutes) in comparison to CAR4 cells (t AICD 328±19 minutes) (Figure 6A). Consistent with the expectation that multi-killers efficiently resist AICD, these T cells from three of four donors displayed low frequencies of cells undergoing AICD (13-25%, Figure 6B). However, multi-killer T cells from the last donor displayed AICD at elevated frequencies (58%) underscoring that the efficiency of multi-killers to execute multiple tumor cells must be evaluated in the context of their ability to resist AICD (Figure 6B). We confirmed that the effector apoptosis that was observed required functional antigenic stimulation by co-incubating CAR8 cells with CD19−EL4 cells within nanowell grids and imaged them using TIMING. The frequency of apoptotic effectors under these conditions was only 4% and this also confirmed that phototoxicity was negligible under the current imaging conditions.
Figure 6. Frequency and kinetics of killer-cell apoptosis are dependent on functional conjugations with multiple NALM-6 tumor cells.
(A) Comparisons of the mean kinetics of effector apoptosis of individual single killer CAR+ T cells (E:T 1:1) with multi-killer CAR+ T cells (E:T 1:2-5). Each circle represents a single-cell; CAR4 cells are represented using grey circles and CAR8 cells are represented using black circles. (B) Frequency of killer-cell apoptosis as a function of tumor cell density.
Significantly, across all four donors, the frequencies of cytolytic CAR+ T cells undergoing AICD was higher at an E:T of 1:1 in comparison to the multi-killer CAR+ T cells, and this effect was more exaggerated with CAR8 cells (Figure 6B). These data may help account for the decrease in number and even disappearance of infused CAR+ T cells when the CD19+ tumor mass is reduced.
DISCUSSION
We implemented a high-throughput single-cell assay (TIMING) to dynamically profile the functionality of CAR+ T cells. Our analyses at the single-cell level demonstrate that much like CAR8 cells, CAR4 cells can directly engage in tumor cell killing, albeit with altered kinetics. We further demonstrate that CAR4 cells can participate in multi-killing via simultaneous conjugation to multiple tumor cells.
At low tumor cell densities (E:T 1:1), the majority of the single killer CAR8 cells were significantly faster in killing tumor cells in comparison to individual CAR4 cells (Figure 4E). By contrast, both single killer CAR8 and CAR4 cells within the S1 subgroup, characterized by their high basal motility, displayed no significant differences in the kinetics of tumor cell killing. Furthermore, in contrast to the rest of the population, effector apoptosis was infrequent amongst CAR8 and CAR4 cells in the S1 subgroup. Collectively, these data suggested that the high basal motility of CAR+ T cells (CAR4 or CAR8) might help identify efficient killers with decreased propensity for AICD.
When interacting with increased numbers of tumor cells (E:T ratios of 1:2 to 1:5), both individual CAR4 and CAR8 cells efficiently conjugated to multiple tumor cells, facilitating multiplexed killing. Comparisons amongst the different tumor cells killed by these individual multi-killer CAR4/CAR8 cells demonstrated that they displayed an essentially unchanged efficiency (tContact) of killing of not only the first and second target killed, but also in comparison to (single-killer) CAR+ T cells that were incubated with only one tumor cell (Figure S20). In comparing CAR4 cells with CAR8 cells however, consistent with the observations at an E:T ratio of 1:1 , CAR4 cells were significantly slower in tumor cell killing. Intracellular staining at the single-cell level indicated that the molecular origin of the differences in kinetic efficiency of the CAR4 and CAR8 cells could be attributed to their GzB content and this was further confirmed by blocking granule exocytosis using EGTA (Figure 5).
For both CAR4 and CAR8 cells, single killer effectors underwent apoptosis at higher frequencies and with faster kinetics in comparison to multi-killer CAR+ T cells (Figures 1 and 4). These data indicate that activation for lysis through multiple targets as opposed to prolonged conjugation with a single target reduces the propensity for effector apoptosis. Although the mechanistic basis for the responsiveness of these T cells to antigen/target density is not known, it is conceivable that the continuous propagation of these cells on irradiated aAPC at defined ratios, allows for balanced activation while minimizing AICD32. Collectively, these data could provide mechanistic insights into observations that infused CAR+ T cells swell in number in response to addressing large numbers of CD19+ tumor cells, but then decline in number as the tumor bioburden is lowered due to the multi-killing by effector T cells6,33.
In aggregate, comparisons of the CAR4 cells and CAR8 cells demonstrate that while CAR4 cells can participate in killing and multi-killing, they do so at slower rates, likely due to the lower GzB content. This decreased kinetic efficiency however is likely a minor disadvantage and is counter balanced by their decreased propensity of these cells to undergo AICD in the absence of help from other cells, as profiled in our nanowell system. Indeed, recent preclinical and clinical data have suggested that complete eradication of established tumors can be accomplished by the adoptive transfer of T cells derived exclusively from CD4+ T cells16-18. Similarly, adoptive transfer of human T helper 17 (TH17) cells has shown preclinical promise for the treatment of ovarian cancer34,35. Although we have focused on the heterogeneity amongst CAR+ T cells, the results presented here are also likely influenced by the underlying heterogeneity in tumor cells. While the expression of CD19 is uniform on the cells used as targets in our assays (Figure S4), it is feasible that there could be subpopulations of tumor cells that are resistant to CAR+ T-cell mediated killing.
Data from clinical trials have also shown a correlation between in vivo persistence of infused CAR+ T cells and patient outcomes36. Significantly, the findings of our short-term TIMING data (12h monitoring) that describes motility and ability to resist AICD as important attributes of functional T cells, is consistent with persistence data obtained in mouse models infusing CD19-specific CAR+ T cells that suggest that these same features are essential for tumor regression37. Motility is likely a key parameter of the efficacy of T-cell therapies and has a significant role in tumor regression. It has been previously demonstrated that cancer cells from B-cell malignancies effectively dampen anti-tumor responses via disruption of actin-based basal T-cell motility in vitro38-40. Second, the negative costimulatory molecules, PD1 and CTLA4 have opposing effects on T-cell motility both in vitro and in vivo41,42. Finally, recent intravital microscopy data from melanoma models in mice have demonstrated that successful therapeutic anti-CTLA4 treatment correlates with greater T-cell motility43.
The variation in the composition of CAR+ T cells within a population of effector cells between donors across samples highlights the challenges in eliciting functional responsiveness in heterogeneous samples. As the field of adoptive immunotherapy takes on the challenge of targeting diseases that vary in burden, biodistribution, and antigen expression and density, it is important that a priori definitions of single-cell potency (proliferation, killing, cytokine secretion etc.) be available. We suggest that identifying/quantifying specific biomarkers of efficacy, as described herein, may enable the manufacture of next-generation CAR+ T cells.
Supplementary Material
ACKNOWLEDGEMENTS
This publication was supported by the NIH R01 (CA174385, CA124782, CA120956, CA141303, CA163587); R33 (CA116127); Cancer Center Core Grant (CA16672); P01 (CA148600); SPORE (CA136411), CPRIT (RP130570); MRA Stewart-Rahr Young Investigator Award; Welch Foundation (E1774); Adee Heebe, Ahuja family; Alex Lemonade Stand Foundation; Burroughs Wellcome Fund; Gillson Longenbaugh Foundation; CLL Global Research Foundation; DoD; National Foundation for Cancer Research; Pediatric Cancer Research Foundation. We thank the flow-cytometry and fingerprinting cores at MDACC, Dr. June at UPenn for assistance with aAPC, Dr. Hackett at U.Minnesota for the SB system; and Drs. McNamara, Mahendra, Kaul and Ramesh for edits. JTA gratefully acknowledges the DOST-UP-ERDT Faculty Development Program.
Footnotes
CONFLICTS OF INTEREST
Dr. Cooper founded and owns InCellerate, Inc. He has patents with Sangamo BioSciences with artificial nucleases. He consults with Targazyme, Inc. (American Stem cells, Inc.), GE Healthcare, Ferring Pharmaceuticals, Inc., Bristol-Myers Squibb. None of these activities had any influence on any part of this study.
REFERENCES
- 1.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–26. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–76. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spear P, Barber A, Sentman CL. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology. 2013;2:e23564. doi: 10.4161/onci.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23:1043–53. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 14.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–50. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–67. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roach KL, King KR, Uygun BE, Kohane IS, Yarmush ML, Toner M. High throughput single cell bioinformatics. Biotechnol Prog. 2009;25:1772–9. doi: 10.1002/btpr.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutsch M, Deutsch A, Shirihai O, Hurevich I, Afrimzon E, Shafran Y, et al. A novel miniature cell retainer for correlative high-content analysis of individual untethered non-adherent cells. Lab Chip. 2006;6:995–1000. doi: 10.1039/b603961h. [DOI] [PubMed] [Google Scholar]
- 21.Liadi I, Roszik J, Romain G, Cooper LJ, Varadarajan N. Quantitative high-throughput single-cell cytotoxicity assay for T cells. J Vis Exp. 2013;(72):e50058. doi: 10.3791/50058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanaka YJ, Berger CT, Sips M, Cheney PC, Alter G, Love JC. Single-cell analysis of the dynamics and functional outcomes of interactions between human natural killer cells and target cells. Integr Biol (Camb) 2012;4:1175–84. doi: 10.1039/c2ib20167d. [DOI] [PubMed] [Google Scholar]
- 23.Vanherberghen B, Olofsson PE, Forslund E, Sternberg-Simon M, Khorshidi MA, Pacouret S, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–34. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- 24.Romain G, Senyukov V, Rey-Villamizar N, Merouane A, Kelton W, Liadi I, et al. Antibody Fc-engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood. 2014;124:3241–9. doi: 10.1182/blood-2014-04-569061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh H, Figliola MJ, Dawson MJ, Huls H, Olivares S, Switzer K, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–27. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G, et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS One. 2013;8:e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izsvak Z, Ivics Z. Sleeping beauty transposition: biology and applications for molecular therapy. Mol Ther. 2004;9:147–56. doi: 10.1016/j.ymthe.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Lex A, Streit M, Schutz HJ, Partl C, Schmalstieg D, Park PJ, et al. StratomeX: Visual Analysis of Large-Scale Heterogeneous Genomics Data for Cancer Subtype Characterization. Comput Graph Forum. 2012;31:1175–84. doi: 10.1111/j.1467-8659.2012.03110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley MH, Forcier T, McAndrew E, Gonzalez M, Chen H, Juelg B, et al. High avidity CD8+ T cells efficiently eliminate motile HIV-infected targets and execute a locally focused program of anti-viral function. PLoS One. 2014;9:e87873. doi: 10.1371/journal.pone.0087873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henkart PA, Millard PJ, Reynolds CW, Henkart MP. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984;160:75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 32.Romer PS, Berr S, Avota E, Na SY, Battaglia M, ten Berge I, et al. Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood. 2011;118:6772–82. doi: 10.1182/blood-2010-12-319780. [DOI] [PubMed] [Google Scholar]
- 33.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–9. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiaii S, Clear AJ, Ramsay AG, Davies D, Sangaralingam A, Lee A, et al. Follicular lymphoma cells induce changes in T-cell gene expression and function: potential impact on survival and risk of transformation. J Clin Oncol. 2013;31:2654–61. doi: 10.1200/JCO.2012.44.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, et al. Follicular lymphoma cells induce T-cell immunological synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–20. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinselmeyer BH, Heydari S, Sacristán C, Nayak D, Cammer M, Herz J, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–74. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 43.Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, Allison JP. Cytotoxic T Lymphocyte Antigen-4 Blockade Enhances Antitumor Immunity by Stimulating Melanoma-Specific T-cell Motility. Cancer Immunol Res. 2014;2:970–80. doi: 10.1158/2326-6066.CIR-14-0104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.