Abstract
The marine cyanobacterium Prochlorococcus is the numerically dominant photosynthetic organism in the oligotrophic oceans, and a model system in marine microbial ecology. Here we report 27 new whole genome sequences (2 complete and closed; 25 of draft quality) of cultured isolates, representing five major phylogenetic clades of Prochlorococcus. The sequenced strains were isolated from diverse regions of the oceans, facilitating studies of the drivers of microbial diversity—both in the lab and in the field. To improve the utility of these genomes for comparative genomics, we also define pre-computed clusters of orthologous groups of proteins (COGs), indicating how genes are distributed among these and other publicly available Prochlorococcus genomes. These data represent a significant expansion of Prochlorococcus reference genomes that are useful for numerous applications in microbial ecology, evolution and oceanography.
Background & Summary
As the smallest (<1 μm diameter) and most abundant (3×1027 cells) photosynthetic organism on the planet1, Prochlorococcus has a unique status in the microbial world. This unicellular marine cyanobacterium is found throughout the euphotic zone of the open ocean between ~45°N and 40°S, where it carries out a notable fraction of global photosynthesis1, 2. The group, which would be considered a single microbial ‘species’ by the traditional measure of >97% 16S rRNA similarity, is composed of multiple phylogenetically distinct clades (Figure 1) (as defined by either rRNA internal transcribed spacer (ITS)3 or whole-genome sequences4) which are physiologically distinct. Adaptations for optimal growth at different light intensities differentiate deeply branching groups of Prochlorococcus into high light (HL) and low light (LL) adapted clades3,5–8.
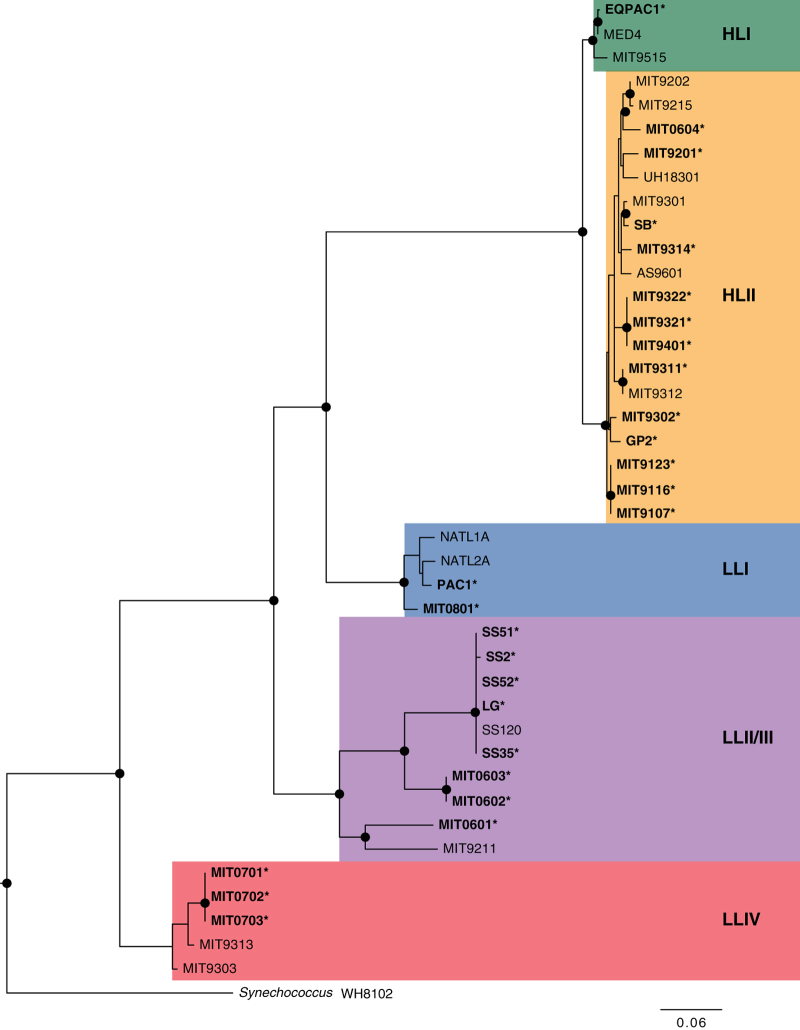
Figure 1.
Prochlorococcus strains sequenced in this work. ITS-based phylogeny of the strains included in this data set (names in bold, with *) in relation to previously sequenced Prochlorococcus. Phylogenetic clade affiliation4,6 is indicated at right; closed circles indicate nodes with bootstrap support >75%. HL—High light adapted; LL—Low light adapted, as determined by physiological studies of some of the isolates3,5,7.
Prochlorococcus have the smallest genomes of any known free-living photosynthetic cell, ranging from ~1.6 to 2.7 Mbp4. While they all share a core set of genes present in all strains, there exists remarkable diversity in gene content among isolates. The group has an ‘open’ pan-genome, i.e. each newly sequenced genome typically contains many new genes never before seen in Prochlorococcus 4. Given the abundance of Prochlorococcus, studies of their genomic and metagenomic features have provided numerous insights into features of ocean ecosystems9–17. In addition, the group has proven to be a valuable system for studying microbial evolution18,19, genome streamlining20,21, and the relationship between genotypic, phenotypic and ecological variation in marine populations3,7,22. Since Prochlorococcus is abundant in surface waters, these reference genomes have also been extremely valuable for interpreting marine metagenomic and metatranscriptomic datasets14,23–28.
To advance our understanding of Prochlorococcus genetic diversity, we sequenced the genomes of 27 Prochlorococcus strains from a variety of ocean environments. The strains sequenced included both previously reported strains as well as eight new isolates (Table 1). The newly isolated strains come from ocean regions that previously only had few or no cultured representatives and substantially expand the number of cultured Prochlorococcus available for five major clades. These results demonstrate the applicability of high-throughput dilution-to-extinction cultivation approaches29 to Prochlorococcus.
Table 1. Origin of the Prochlorococcus strains sequenced in this study.
| Strain | Alternate Name | Ecotype/Clade 4,57 | Isolation location | Isolation (Lat/Lon) | Isolation depth (m) | Isolation date | Strain reference |
|---|---|---|---|---|---|---|---|
| EQPAC1 | RCC278 | eMED4/HLI | Equatorial Pacific | 0°N 180°W | 30 | Roscoff Culture Collection | |
| GP2 | eMIT9312/HLII | Western Pacific | 8°N 136°E | 150 | Sep-1992 | 32 | |
| MIT0604 | eMIT9312/HLII | Station ALOHA/North Pacific | 22.75°N 158°W | 175 | May-2006 | This work | |
| MIT9107 | eMIT9312/HLII | Tropical Pacific | 15°S 135°W | 25 | 8-Aug-1991 | 33 | |
| MIT9116 | eMIT9312/HLII | Tropical Pacific | 15°S 135°W | 25 | 8-Aug-1991 | 6 | |
| MIT9123 | eMIT9312/HLII | Tropical Pacific | 15°S 135°W | 25 | 8-Aug-1991 | 6 | |
| MIT9201 | eMIT9312/HLII | Tropical Pacific | 12°S 145.42°W | Surface | 26-Sep-1992 | 5 | |
| MIT9302 | eMIT9312/HLII | Sargasso Sea | 34.76°N 66.19°W | 100 | 15-Jul-1993 | 3 | |
| MIT9311 | eMIT9312/HLII | Gulf stream | 37.51°N 64.24°W | 135 | 17-Jul-1993 | 6 | |
| MIT9314 | eMIT9312/HLII | Gulf stream | 37.51°N 64.24°W | 180 | 17-Jul-1993 | 6 | |
| MIT9321 | eMIT9312/HLII | Equatorial Pacific | 1°N 92°W | 50 | 12-Nov-1993 | 6 | |
| MIT9322 | eMIT9312/HLII | Equatorial Pacific | 0.27°N 93°W | Surface | 16-Nov-1993 | 6 | |
| MIT9401 | eMIT9312/HLII | Sargasso Sea | 35.5°N 70.4°W | Surface | May-1994 | 6 | |
| SB | eMIT9312/HLII | Western Pacific | 35°N 138.3°E | 40 | 1-Oct-1992 | 32 | |
| MIT0801 | HTCC 1603 | eNATL/LLI | BATS/Sargasso Sea | 31.67°N 64.17°W | 40 | 25-Mar-2008 | This work |
| PAC1 | eNATL/LLI | Station ALOHA/North Pacific | 22.75°N 158°W | 100 | 1992 | 34,35 | |
| LG | eSS120/LLII,III | Sargasso Sea | 28.98°N 64.35°W | 120 | 30-May-1988 | 36 | |
| MIT0601 | eMIT9211/LLII,III | Station ALOHA/North Pacific | 22.75°N 158°W | 125 | 17-Nov-2006 | This work | |
| MIT0602 | eSS120/LLII,III | Station ALOHA/North Pacific | 22.75°N 158°W | 125 | 17-Nov-2006 | This work | |
| MIT0603 | eSS120/LLII,III | Station ALOHA/North Pacific | 22.75°N 158°W | 125 | 17-Nov-2006 | This work | |
| SS2 | eSS120/LLII,III | Sargasso Sea | 28.98°N 64.35°W | 120 | 30-May-1988 | 6 | |
| SS35 | eSS120/LLII,III | Sargasso Sea | 28.98°N 64.35°W | 120 | 30-May-1988 | 6 | |
| SS51 | eSS120/LLII,III | Sargasso Sea | 28.98°N 64.35°W | 120 | 30-May-1988 | 6 | |
| SS52 | eSS120/LLII,III | Sargasso Sea | 28.98°N 64.35°W | 120 | 30-May-1988 | 6 | |
| MIT0701 | HTCC 1600 | eMIT9313/LLIV | South Atlantic | 13.45°S 0.04°W | 150 | 1-Dec-2007 | This work |
| MIT0702 | HTCC 1601 | eMIT9313/LLIV | South Atlantic | 13.45°S 0.04°W | 150 | 1-Dec-2007 | This work |
| MIT0703 | HTCC 1602 | eMIT9313/LLIV | South Atlantic | 13.45°S 0.04°W | 150 | 1-Dec-2007 | This work |
The genome sequences reported here represent a notable increase in the number of genome sequences available from the major phylogenetic clades with existing cultured representatives. While many genomes differed greatly in gene content, other sets are very closely related and differ primarily by single nucleotide polymorphisms (e.g., LG, SS2, SS35, SS51, SS52, SS120; and MIT0701, MIT0702, and MIT0703). Thus, this dataset encompasses a broad range of pairwise genomic diversity among Prochlorococcus strains.
Most genomes were sequenced to draft status; two were closed (Table 2). We used two annotation methods to identify the potential functions of genes in the genomes. Genes were first called and annotated by the RAST pipeline30. To expand on these predictions—especially for the myriad genes of unknown function—we also derived annotations from an independent pipeline, Argot231. To facilitate the utility of these genomes for comparative genomics and evolutionary studies, we define a set of pre-computed orthologous gene clusters for Prochlorococcus. All cluster data are supplied in this data set (Data Citation 1 and Data Citation 2).
Table 2. Genome characteristics and assembly statistics.
| Strain | Clade 4 | Assembly size (bp) | %GC | No. contigs | N50 (bp) | No. coding sequences | NCBI accession* |
|---|---|---|---|---|---|---|---|
| *For the Whole Genome Shotgun projects deposited at DDBJ/EMBL/GenBank: the version described in this paper is version JN**01000000. | |||||||
| EQPAC1 | HLI | 1,654,739 | 30.8 | 8 | 328,627 | 1,954 | JNAG00000000 |
| GP2 | HLII | 1,624,310 | 31.2 | 11 | 416,038 | 1,884 | JNAH00000000 |
| MIT0604 | HLII | 1,780,061 | 31.2 | 1 | 1,780,061 | 2,085 | CP007753 |
| MIT9107 | HLII | 1,699,937 | 31.0 | 13 | 170,362 | 1,991 | JNAI00000000 |
| MIT9116 | HLII | 1,685,398 | 31.0 | 22 | 117,620 | 1,972 | JNAJ00000000 |
| MIT9123 | HLII | 1,697,748 | 31.0 | 18 | 137,374 | 2,005 | JNAK00000000 |
| MIT9201 | HLII | 1,672,416 | 31.3 | 21 | 145,955 | 1,989 | JNAL00000000 |
| MIT9302 | HLII | 1,745,343 | 31.1 | 17 | 242,124 | 2,015 | JNAM00000000 |
| MIT9311 | HLII | 1,711,064 | 31.2 | 17 | 189,094 | 1,983 | JNAN00000000 |
| MIT9314 | HLII | 1,690,556 | 31.2 | 16 | 221,824 | 1,990 | JNAO00000000 |
| MIT9321 | HLII | 1,658,664 | 31.2 | 10 | 259,210 | 1,956 | JNAP00000000 |
| MIT9322 | HLII | 1,657,550 | 31.2 | 11 | 367,597 | 1,959 | JNAQ00000000 |
| MIT9401 | HLII | 1,666,808 | 31.2 | 17 | 110,519 | 1,972 | JNAR00000000 |
| SB | HLII | 1,669,823 | 31.5 | 4 | 1,237,529 | 1,933 | JNAS00000000 |
| MIT0801 | LLI | 1,929,203 | 34.9 | 1 | 1,929,203 | 2,287 | CP007754 |
| PAC1 | LLI | 1,841,163 | 35.1 | 20 | 182,484 | 2,264 | JNAX00000000 |
| LG | LLII,III | 1,754,063 | 36.4 | 14 | 326,623 | 1,973 | JNAT00000000 |
| MIT0601 | LLII,III | 1,707,342 | 37.0 | 6 | 547,047 | 1,934 | JNAU00000000 |
| MIT0602 | LLII,III | 1,750,918 | 36.3 | 9 | 511,704 | 1,998 | JNAV00000000 |
| MIT0603 | LLII,III | 1,752,482 | 36.3 | 7 | 434,668 | 2,015 | JNAW00000000 |
| SS2 | LLII,III | 1,752,772 | 36.4 | 19 | 187,268 | 1,989 | JNAY00000000 |
| SS35 | LLII,III | 1,751,015 | 36.4 | 9 | 446,270 | 1,977 | JNAZ00000000 |
| SS51 | LLII,III | 1,746,977 | 36.4 | 12 | 232,789 | 1,974 | JNBD00000000 |
| SS52 | LLII,III | 1,754,053 | 36.4 | 22 | 124,224 | 1,987 | JNBE00000000 |
| MIT0701 | LLIV | 2,592,571 | 50.6 | 53 | 84,463 | 3,079 | JNBA00000000 |
| MIT0702 | LLIV | 2,583,057 | 50.6 | 61 | 76,101 | 3,066 | JNBB00000000 |
| MIT0703 | LLIV | 2,575,057 | 50.6 | 61 | 81,186 | 3,054 | JNBC00000000 |
These genomes should be useful to researchers interested in many aspects of marine microbial ecology and evolution. Since the genomes are from cultured isolates, hypotheses generated from these data can be tested in laboratory experiments. The genomes will also greatly facilitate the interpretation of transcriptomic and proteomic studies, as well as meta-‘omic’ data from field studies where Prochlorococcus is a dominant phototroph.
Methods
Culturing and strain isolations
Many of the strains sequenced have been previously described3,5,6,32–36 (Table 1); 8 are reported here for the first time. All cultures were unialgal; this was initially determined crudely by flow cytometry profiles, and then more specifically by confirming the presence of only one cyanobacterial 16S rRNA ITS sequence in the culture. All cultures except SB and MIT0604 contained heterotrophic bacteria. Cultures were maintained in acid-washed glassware in Pro99 media37 prepared with 0.2 μm filtered, autoclaved seawater collected from Vineyard Sound, MA or the Sargasso Sea under either a 14:10 light:dark cycle at 24 °C or constant light flux at 21 °C. Light levels were 30–40 μmol Q m−2 s−1 for high-light adapted strains, and 10–20 μmol Q m−2 s−1 for low-light adapted strains.
MIT0601, MIT0602, MIT0603, and MIT0604 were derived from enrichment cultures initiated with seawater obtained from the North Pacific Ocean at Station ALOHA (22.75°N, 158°W) on Hawai’i Ocean Time-series (HOT) cruise 181. The seawater was amended with nitrogen, phosphorous and trace metals (PRO2 nutrient additions37, except all nitrogen sources were replaced by 0.217 mM sodium nitrate).
Strains MIT0701, MIT0702, and MIT0703 were isolated from the South Atlantic (CoFeMUG cruise KN192-05, station 13, 13.45 °S, 0.04 °W) at 150 m using a high throughput culturing method29 adapted for phototrophs. The seawater used for isolations was first filtered through a 1 μm filter with no amendments and kept in the dark at 18–20 °C for 21 days. The total red fluorescing phytoplankton population (1×105 cells ml−1 determined with a Guava EasyCyte flow cytometer) was diluted in PRO3V media37 made with the same South Atlantic water that had been filtered through a 0.1 μm Supor 142 mm filter, then autoclaved to sterilize. This media contained 100 μM NH4Cl, 10 μM NaH2PO4, PRO2 trace metals37 and f/2 vitamins (0.1 μg l−1 cyanocobalamin, 20 g l−1 thiamin and 1 μg l−1 biotin38,39). Ten cells were dispensed into 1 ml volumes in a 48-well polystyrene multiwell culture plate and incubated at 20 °C in ~20 μmol Q m−2 s−1 (14:10 light:dark) for 2 months.
MIT0801 was isolated in a similar manner, but from seawater obtained from 40 m depth at the Bermuda Atlantic Time-series station (BATS; 31.67 °N, 64.16 °W) that had been sitting in the dark for 5 days. The same PRO3V media recipe was made with 0.1 μm filtered and autoclaved BATS seawater, and 2.5 cells (on average) were dispensed in 5 ml volume in Teflon plates (prepared as described29). Cells were detected within 1 month of enrichment.
DNA sequencing and assembly
Genomes were sequenced from genomic DNA collected from 20 ml laboratory cultures. Cells were collected by centrifugation (10,000g, 10 min), the pellet transferred into a 2 ml tube and frozen at −80 °C. Genomic DNA was isolated using the QIAamp DNA mini kit (Qiagen). 2 μg of DNA was then used to construct an Illumina sequencing library as previously described40, except that the bead:sample ratios in the double solid phase reversible immobilization (dSPRI) size-selection step were 0.7 followed by 0.15, resulting in fragments with an average size of ~340 bp (range: 200–600 bp). PAC1 and EQPAC1 libraries were constructed using dSPRI bead:sample ratios of 0.9 followed by 0.21, yielding an average size of ~220 bp. DNA libraries were sequenced on an Illumina GAIIx, producing 200+200 nt paired reads, at the MIT BioMicro Center. An average of 1.6 million paired-end reads were obtained for each genome.
Low quality regions of sequencing data were removed from the raw Illumina data using quality_trim (V3.2, from the CLC Assembly Cell package; CLC bio) with default settings (at least 50% of the read must be of a minimum quality of 20). Paired-end reads were overlapped using the SHE-RA algorithm41, keeping any resulting overlapping sequences with an overlap score >0.5. For all genomes except PAC1 and EQPAC1, the overlapped reads, as well as the trimmed paired-end reads that did not overlap, were assembled using the Newbler assembler (V2.6; 454/Roche) with the following parameters: ‘-e 200 –rip.’ Contigs <1 Kbp were discarded at this stage.
Reads for PAC1 and EQPAC1 were assembled using clc_novo_assemble (V3.2, from the CLC Assembly Cell package; CLC bio) with a minimum contig length of 500 bp and automatic wordsize determination enabled. These initial contigs were searched against a custom database of marine microbial genomes9 using BLAST42 to identify contigs with a closest match to Prochlorococcus. Sequencing reads belonging to the putative Prochlorococcus contigs were then identified by mapping the raw sequences to these contigs using clc_ref_asssemble_long (CLC bio). The Prochlorococcus-like reads were then re-assembled using clc_novo_assemble using the same parameters as above to produce the final assembly, now largely free of heterotrophic sequences.
MIT0604 and MIT0801 were completed to finished quality with no gaps by directed PCR reactions to sequence contig junctions, combined with Pacific Biosciences long sequencing reads. Contigs were ordered into putative scaffolds based on their similarity to closely related closed Prochlorococcus genomes, as determined by Mauve43. PCR primers specific to the ends of putatively adjacent contigs were designed and used to amplify the junctions between contigs. Purified PCR products were sequenced by Sanger chemistry at the MGH DNA core facility, and the resulting sequences used to join contigs in Consed44. This resulted in a highly improved but still incomplete assembly. To span difficult repeat regions in MIT0801, we obtained long Pacific Biosciences sequences. We obtained DNA from 25 ml cultures using the Epicentre Masterpure kit (Epicentre) and sequenced this at the Yale Center for Genome Analysis. We combined this set of long but low quality reads with the high quality Illumina short reads obtained previously using the PacBioToCA software45, to produce assemblies with a reduced number of contigs. These contigs were aligned to the PCR-improved contigs described above, and the final gaps were closed with a small number of additional directed PCR reactions (as described above) using the Geneious sequence analysis package (V6.1, Biomatters), until the genomes were closed.
As most of the Prochlorococcus cultures sequenced were known to contain heterotrophs, we identified the most ‘Prochlorococcus-like’ contigs from non-axenic cultures by searching each resulting contig against a custom database of sequenced marine microbial genomes9 using BLAST42. Contigs with a best match to a non-Prochlorococcus genome were removed from the assembly. Subsequent examination of these contig sets indicated that a number of shorter sequences (generally <10 kbp) with significant heterotroph-like stretches had passed through the initial filtering steps. To remove these questionable contigs from the assemblies, we manually examined each <10 kbp contig using the RAST annotation server (see below), and only kept those contigs with clear homology to previously sequenced and closed Prochlorococcus or Synechococcus genomes. Although these filtering steps may have removed a small amount of true Prochlorococcus sequence from the final assembly, we considered missing a few genes preferable to misrepresenting heterotroph sequences as Prochlorococcus.
Examination of the non-cyanobacterial 16S rRNA genes found within these data indicate that the most abundant heterotrophs in the cultures were members of the Alteromonadales, Flavobacteriales, Rhodospirillales, Halomonadaceae, and Sphingobacteriales. We have included a separate data file containing all of the assembled contigs—including those from co-cultured heterotrophs—for anyone who is interested (Data File 4).
Genome annotation
The assembled contigs for each genome were annotated using the RAST server30 against FIGfam release 49. Additional functional annotation for all genes called by RAST were generated by the Argot2 server31, using default settings.
To confirm the rRNA-based phylogeny of these strains, rRNA ITS sequences were aligned in ARB46 and maximum likelihood phylogenies calculated in PhyML version 2012041247, using the HKY85 model of nucleotide substitution, a fixed proportion of invariable sites, and non-parametric bootstrap analysis with 100 replicates.
Clusters of orthologous groups of proteins (COGs) were computed, as described elsewhere48, on a data set comprised of previously sequenced Prochlorococcus and Synechococcus strains4,10,16,17,49–53, the new Prochlorococcus genomes described here, 11 Prochlorococcus single-cell genomes12 and two consensus metagenomic assemblies14 (Data Citation 1). To facilitate comparisons among genomes, we re-annotated 16 previously sequenced Prochlorococcus genomes (Table 3) with the RAST pipeline as described above; this ensured that a uniform methodology for gene calling and functional annotation was used. Single cell genomes12 were not re-annotated due to difficulties encountered using this pipeline on such fragmented contigs; instead, we utilized the ORFs previously defined in GenBank. Detailed information regarding these updated annotations is provided (Data Citation 1 and Data Citation 2).
Table 3. Previously sequenced Prochlorococcus genomes included in the cyanobacterial clusters of orthologous groups of proteins (CyCOG) definitions.
| Name | Genome source | Clade | Assembly size (bp) | %GC | No. coding sequences* | NCBI accession | Sequence reference |
|---|---|---|---|---|---|---|---|
| *For the cultured isolate and metagenomic assembly genomes, this value represents the number of coding sequences as predicted in this study using the RAST pipeline; these values may differ from those previously published for this reason. Re-annotation data is included in this dataset (Data Citation 1 and Data Citation 2). | |||||||
| MED4 | Cultured isolate | HLI | 1,657,990 | 30.8 | 1,959 | BX548174 | 10 |
| MIT9515 | Cultured isolate | HLI | 1,704,176 | 30.8 | 1,951 | CP000552 | 4 |
| AS9601 | Cultured isolate | HLII | 1,669,886 | 31.3 | 1,944 | CP000551 | 4 |
| MIT9202 | Cultured isolate | HLII | 1,691,453 | 31.1 | 2,000 | DS999537 | 49 |
| MIT9215 | Cultured isolate | HLII | 1,738,790 | 31.1 | 2,035 | CP000825 | 4 |
| MIT9301 | Cultured isolate | HLII | 1,641,879 | 31.3 | 1,925 | CP000576 | 4 |
| MIT9312 | Cultured isolate | HLII | 1,709,204 | 31.2 | 1,982 | CP000111 | 16 |
| UH18301 | Cultured isolate | HLII | 1,654,648 | 31.2 | 1,947 | PRJNA47033 | 50 |
| W6 | Single cell amplified genome | HLII | 385,307 | 31.3 | 646 | ALPK00000000 | 12 |
| HNLC2 | Metagenomic assembly | HLIII | 1,484,494 | 30.3 | 1,701 | GL947595 | 14 |
| W3 | Single cell amplified genome | HLIII | 339,045 | 30.7 | 529 | ALPC00000000 | 12 |
| W5 | Single cell amplified genome | HLIII | 99,467 | 29.8 | 212 | ALPL00000000 | 12 |
| W7 | Single cell amplified genome | HLIII | 905,221 | 30.7 | 989 | ALPE00000000 | 12 |
| W8 | Single cell amplified genome | HLIII | 841,756 | 31.4 | 917 | ALPF00000000 | 12 |
| W9 | Single cell amplified genome | HLIII | 420,150 | 30.7 | 638 | ALPG00000000 | 12 |
| HNLC1 | Metagenomic assembly | HLIV | 1,569,623 | 29.8 | 1,830 | GL947594 | 14 |
| W10 | Single cell amplified genome | HLIV | 561,998 | 30.8 | 892 | ALPH00000000 | 12 |
| W11 | Single cell amplified genome | HLIV | 766,829 | 30.6 | 929 | ALPI00000000 | 12 |
| W12 | Single cell amplified genome | HLIV | 423,437 | 29.6 | 602 | ALPJ00000000 | 12 |
| W2 | Single cell amplified genome | HLIV | 1,266,767 | 30.5 | 1,374 | ALPB00000000 | 12 |
| W4 | Single cell amplified genome | HLIV | 765,485 | 29.9 | 819 | ALPD00000000 | 12 |
| NATL1A | Cultured isolate | LLI | 1,864,731 | 35.0 | 2,242 | CP000553 | 4 |
| NATL2A | Cultured isolate | LLI | 1,842,899 | 35.1 | 2,194 | CP000095 | 4 |
| MIT9211 | Cultured isolate | LLII,III | 1,688,963 | 38.0 | 1,943 | CP000878 | 4 |
| SS120 | Cultured isolate | LLII,III | 1,751,080 | 36.4 | 1,973 | AE017126 | 17 |
| MIT9303 | Cultured isolate | LLIV | 2,682,675 | 50.0 | 3,253 | CP000554 | 4 |
| MIT9313 | Cultured isolate | LLIV | 2,410,873 | 50.7 | 2,993 | BX548175 | 10 |
Orthologous gene clusters were defined based on reciprocal best blastp scores (with an e-value cutoff of 1e−5); the sequence alignment length had to be at least 75% of the shorter protein, with at least a 35% identity. Additional orthologous genes that did not pass this criterion were added to clusters based on HMM profiles constructed from automated MUSCLE54 alignments of orthologous sequences within each cluster using HMMER55. The clusters described here are noted as ‘V4’ CyCOGs in the associated Data Records and on the ProPortal website48 (Data Citation 1).
Data Records
The complete dataset is available from the Prochlorococcus Portal website (Data Citation 1) and Dryad (Data Citation 2). The 27 Prochlorococcus genome sequences have also been deposited at DDBJ/EMBL/GenBank (3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29) under the accession numbers indicated in Table 2.
Datasets deposited at Dryad and ProPortal
Sequence, gene annotations, and COG definitions for Prochlorococcus genomes.
File 1—Tab-delimited file containing cluster assignments and annotation metadata for genes in the newly sequenced Prochlorococcus genomes described in this work, as well as previously published genomes. Columns are as follows:
Genome
The Prochlorococcus strain where the gene is found.
Gene ID
Unique ID for each Prochlorococcus gene, of the format ‘P<strain>_####’. Note that, due to the re-annotation of previously published genomes, these names (and the underlying gene boundaries) may not necessarily correspond to those in Genbank.
NCBI ID
For the new genome sequences presented here, the systematic NCBI locus_tag identifier for that gene. For previously published genomes, this column contains the corresponding Genbank locus ID (noted as an ‘Alternative locus ID’ for strains MED4, SS120 and MIT9313 in Genbank) from Kettler et al. (2007)4.
V1 CyCOG
Where applicable, the cyanobacterial cluster of orthologous groups of proteins (CyCOG) definition from Kettler et al. (2007)4.
V3 CyCOG
Where applicable, the CyCOG definition from Kelly et al. (2013)56.
V4 CyCOG
Number indicating the CyCOG to which this gene belongs, as defined in this work.
RAST annotation
Predicted functional annotation description, as supplied by the RAST annotation pipeline. Note that this text may differ slightly from the annotation in Genbank, due to changes imposed by NCBI annotation formatting guidelines.
GO annotation
Gene Ontology categorization for the gene, when available.
Argot2 annotation
Functional annotation prediction from the Argot2 pipeline, when available.
File 2 – Full RAST gene/protein sequence and annotation results. ZIP format file archive of individual tab-delimited files. Files are supplied for the new genome sequences presented here, as well as re-annotations of previously published genomes included in the CyCOG definitions. Columns are as follows:
contig_id
The name of the sequence contig on which the gene is found.
gene_id
The unique Gene ID code for that feature.
feature_id
Unique RAST-generated identifier for that feature.
type
peg: protein encoding gene; rna: RNA molecule.
location
Ordered location code for the position on the genome merging contig_id, start, and stop position.
start
Start location on contig, bp.
stop
Stop location on contig, bp.
strand
Orientation of gene on contig (+: on forward strand; −: on reverse).
function
The predicted function of the feature, if known.
aliases
Alternative names for the predicted function.
figfam
FigFAM membership for that feature.
evidence_codes
Code indicating the reason for the annotation. See http://www.nmpdr.org/FIG/wiki/view.cgi/FIG/EvidenceCode for more details.
nucleotide_sequence
The nucleotide sequence of the predicted gene.
aa_sequence
The protein (amino acid) sequence of the predicted gene.
File 3 – Set of nucleotide FASTA-formatted files containing the new Prochlorococcus genome assemblies described in this work.
File 4 – Set of nucleotide FASTA files containing all assembled contigs (>500 bp) from each culture (i.e., both Prochlorococcus and heterotrophs) sequenced in this work. Each file contains the set of contigs assembled from the raw sequencing data, before any filtering to separate Prochlorococcus from heterotroph contigs. These files are provided for reference, but due to the known heterotroph sequences in these files, they should be used with caution.
File 5 – Set of nucleotide FASTA files containing the predicted nucleotide sequence for all open reading frames (ORFs) in each genome. This file includes ORFs from both the new genomes presented here as well as the re-annotation of previously released Prochlorococcus genomes.
File 6 – Set of protein FASTA files containing the predicted amino acid translation for all ORFs in each genome. This file includes ORFs from both the new genomes presented here as well as the re-annotation of previously released Prochlorococcus genomes.
Technical Validation
Phylogenetic analysis of the ITS sequences obtained from these cultured isolate genomes (Figure 1) group these strains into the expected clades57 as previously determined from directed sequencing of the ITS sequences6. We were only able to obtain a single cyanobacterial ITS sequence from the assembled genome contigs, again consistent with these strains being unialgal. Prochlorococcus genome size and %GC content are typically quite similar for strains found within the same ITS-defined clade4, and both the draft and closed genomes are consistent with previously sequenced strains for these measures as well (Table 2).
The quality of the genome assemblies was assessed in multiple ways. Re-mapping of the original Illumina sequencing reads to the final assembled contigs showed that the reads were distributed evenly along the length of the assembly, ruling out some categories of major assembly errors (such as duplicated regions). Whole-genome alignments of contigs against closely related closed reference Prochlorococcus genomes indicated that the overall gene order of these contigs was broadly consistent with known sequences, indicating that the sequences do not contain obvious chimeras or other artifacts. We also estimated the completeness of the draft genomes by examining the core gene content of the strains, based on the set of genes shared by all closed Prochlorococcus genomes. We found that all of the draft genome assemblies contained >98% of the genes universally present in the 13 previously published closed Prochlorococcus genomes, indicating that these contigs represent most (or perhaps all) of the genome sequence.
The final closed sequences of the MIT0604 and MIT0801 genomes were verified in two additional ways. First, we compared the experimentally observed PCR product sizes from directed contig joining reactions to the distances predicted from the final assembled sequence to confirm the assembly. Second, we mapped the original (quality trimmed) Illumina sequencing reads against the final assembly. These alignments indicated that the final closed assembly was fully consistent with the original short-read sequence data. In addition, we confirmed that the per-base SNP frequency was not above the expected error frequency.
Additional information
How to cite this article: Biller, S. J. et al. Genomes of diverse isolates of the marine cyanobacterium Prochlorococcus. Sci. Data 1:140034 doi: 10.1038/sdata.2014.34 (2014).
Supplementary Material
Acknowledgments
The authors are grateful to Allison Coe for careful maintenance of the MIT Prochlorococcus culture collection. We thank Luke Thompson, as well as the HOT and BATS teams, for assistance with field sampling. This work was supported in part by the Gordon and Betty Moore Foundation through Grant GBMF #495.01 and the National Science Foundation through grants OCE-1153588, OCE-0425602 and DBI-0424599, the NSF Center for Microbial Oceanography: Research and Education (C-MORE) to S.W.C. L.R.M. was supported by a NSF-ROA Supplement to NSF grant OCE-0806455 (to S.J.G.); L.R.M. and K.H.R.-J. were also supported by NSF OCE-0851288. G.R. was supported by NSF grant OCE-0723866.
Footnotes
The authors declare no competing financial interests.
Data Citations
- Biller S. J. 2014. Prochlorococcus Portal. http://proportal.mit.edu/
- Biller S. J. 2014. Dryad. http://dx.doi.org/10.5061/dryad.k282c
- Biller S. J. 2014. Genbank. JNAG00000000
- Biller S. J. 2014. Genbank. JNAH00000000
- Biller S. J. 2014. Genbank. CP007753
- Biller S. J. 2014. Genbank. JNAI00000000
- Biller S. J. 2014. Genbank. JNAJ00000000
- Biller S. J. 2014. Genbank. JNAK00000000
- Biller S. J. 2014. Genbank. JNAL00000000
- Biller S. J. 2014. Genbank. JNAM00000000
- Biller S. J. 2014. Genbank. JNAN00000000
- Biller S. J. 2014. Genbank. JNAO00000000
- Biller S. J. 2014. Genbank. JNAP00000000
- Biller S. J. 2014. Genbank. JNAQ00000000
- Biller S. J. 2014. Genbank. JNAR00000000
- Biller S. J. 2014. Genbank. JNAS00000000
- Biller S. J. 2014. Genbank. CP007754
- Biller S. J. 2014. Genbank. JNAX00000000
- Biller S. J. 2014. Genbank. JNAT0000000
- Biller S. J. 2014. Genbank. JNAU00000000
- Biller S. J. 2014. Genbank. JNAV00000000
- Biller S. J. 2014. Genbank. JNAW00000000
- Biller S. J. 2014. Genbank. JNAY00000000
- Biller S. J. 2014. Genbank. JNAZ00000000
- Biller S. J. 2014. Genbank. JNBD00000000
- Biller S. J. 2014. Genbank. JNBE00000000
- Biller S. J. 2014. Genbank. JNBA00000000
- Biller S. J. 2014. Genbank. JNBB00000000
- Biller S. J. 2014. Genbank. JNBC00000000
References
- Flombaum P. et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl Acad. Sci. 110, 9824–9829 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partensky F., Hess W. R. & Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol. Mol. Biol. Rev. 63, 106–127 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. R., Rocap G. & Chisholm S. W. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature 393, 464–467 (1998). [DOI] [PubMed] [Google Scholar]
- Kettler G. C. et al. Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genetics 3, e231 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. & Chisholm S. Photophysiology of the marine cyanobacterium Prochlorococcus: ecotypic differences among cultured isolates. Limnol. and Oceanogr. 44, 628–638 (1999). [Google Scholar]
- Rocap G., Distel D. L., Waterbury J. B. & Chisholm S. W. Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl. Environ. Microbiol. 68, 1180–1191 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser E. R. et al. Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol. Oceanogr. 52, 2205–2220 (2007). [Google Scholar]
- Johnson Z. I. et al. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311, 1737–1740 (2006). [DOI] [PubMed] [Google Scholar]
- Coleman M. L. & Chisholm S. W. Ecosystem-specific selection pressures revealed through comparative population genomics. Proc. Natl. Acad. Sci. 107, 18634–18639 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocap G. et al. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 424, 1042–1047 (2003). [DOI] [PubMed] [Google Scholar]
- Martiny A. C., Huang Y. & Li W. Occurrence of phosphate acquisition genes in Prochlorococcus cells from different ocean regions. Environ. Microbiol. 11, 1340–1347 (2009). [DOI] [PubMed] [Google Scholar]
- Malmstrom R. R. et al. Ecology of uncultured Prochlorococcus clades revealed through single-cell genomics and biogeographic analysis. ISME J. 7, 184–198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Tyson G. W. & Delong E. F. Widespread known and novel phosphonate utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ. Microbiol. 12, 222–238 (2010). [DOI] [PubMed] [Google Scholar]
- Rusch D. B., Martiny A. C., Dupont C. L., Halpern A. L. & Venter J. C. Characterization of Prochlorococcus clades from iron-depleted oceanic regions. Proc. Natl Acad. Sci. 107, 16184–16189 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny A. C., Coleman M. L. & Chisholm S. W. Phosphate acquisition genes in Prochlorococcus ecotypes: evidence for genome-wide adaptation. Proc. Nat. Acad. Sci. 103, 12552–12557 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. L. et al. Genomic islands and the ecology and evolution of Prochlorococcus. Science 311, 1768–1770 (2006). [DOI] [PubMed] [Google Scholar]
- Dufresne A. et al. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc. Natl Acad. Sci. 100, 10020–10025 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaxybayeva O., Doolittle W. F., Papke R. T. & Gogarten J. P. Intertwined evolutionary histories of marine Synechococcus and Prochlorococcus marinus. Genome Biol. Evol. 1, 325–339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumdicker F., Hess W. R. & Pfaffelhuber P. The infinitely many genes model for the distributed genome of bacteria. Genome Biol. Evol. 4, 443–456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne A., Garczarek L. & Partensky F. Accelerated evolution associated with genome reduction in a free-living prokaryote. Genome Biol. 6, R14 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z. & Blanchard J. L. Strong genome-wide selection early in the evolution of Prochlorococcus resulted in a reduced genome through the loss of a large number of small effect genes. PLoS ONE 9, e88837 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashtan N. et al. Single-cell genomics reveals hundreds of coexisting subpopulations in wild. Prochlorococcus. Science 344, 416–420 (2014). [DOI] [PubMed] [Google Scholar]
- Venter J. C. et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304, 66–74 (2004). [DOI] [PubMed] [Google Scholar]
- Rusch D. B. et al. The Sorcerer II Global Ocean Sampling expedition: northwest Atlantic through eastern tropical Pacific. PLoS Biol 5, e77–e431 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias-Lopez J. et al. Microbial community gene expression in ocean surface waters. Proc. Natl. Acad. Sci. 105, 3805–3810 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R. R. et al. Metagenome of the Mediterranean deep chlorophyll maximum studied by direct and fosmid library 454 pyrosequencing. ISME J. 4, 1154–1166 (2010). [DOI] [PubMed] [Google Scholar]
- Shi Y., Tyson G. W., Eppley J. M. & Delong E. F. Integrated metatranscriptomic and metagenomic analyses of stratified microbial assemblages in the open ocean. ISME J. 5, 999–1013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretsky R. S. et al. Comparative day/night metatranscriptomic analysis of microbial communities in the North Pacific subtropical gyre. Environ. Microbiol. 11, 1358–1375 (2009). [DOI] [PubMed] [Google Scholar]
- Stingl U., Tripp H. J. & Giovannoni S. J. Improvements of high-throughput culturing yielded novel SAR11 strains and other abundant marine bacteria from the Oregon coast and the Bermuda Atlantic Time Series study site. ISME J. 1, 361–371 (2007). [DOI] [PubMed] [Google Scholar]
- Aziz R. K. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9, 75 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falda M. et al. Argot2: a large scale function prediction tool relying on semantic similarity of weighted Gene Ontology terms. BMC Bioinformatics 13, S14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A., Nishijima M. & Maruyama T. Seasonal appearance of Prochlorococcus in Suruga Bay, Japan in 1992–1993. J. Oceanogr. 51, 289–300 (1995). [Google Scholar]
- Urbach E., Scanlan D. J., Distel D. L., Waterbury J. B. & Chisholm S. W. Rapid diversification of marine Picophytoplankton with dissimilar light-harvesting structures inferred from sequences of Prochlorococcus and Synechococcus (Cyanobacteria). J. Mol. Evol. 46, 188–201 (1998). [DOI] [PubMed] [Google Scholar]
- Parpais J., Marie D., Partensky F., Morin P. & Vaulot D. Effect of phosphorus starvation on the cell cycle of the photosynthetic prokaryote Prochlorococcus spp. Mar. Ecol. Prog. Ser. 132, 265–274 (1996). [Google Scholar]
- Penno S., Campbell L. & Hess W. R. Presence of phycoerythrin in two strains of Prochlorococcus (Cyanobacteria) isolated from the subtropical North Pacific Ocean. J. Phycol. 36, 723–729 (2000). [DOI] [PubMed] [Google Scholar]
- Chisholm S. W. et al. Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chlorophyll a and b . Arch. Microbiol. 157, 297–300 (1992). [Google Scholar]
- Moore L. et al. Culturing the marine cyanobacterium Prochlorococcus. Limnol. Oceanogr. Methods 5, 353–362 (2007). [Google Scholar]
- Guillard R. R. & Ryther J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 8, 229–239 (1962). [DOI] [PubMed] [Google Scholar]
- Guillard R. R. in Culture of Marine Invertebrate Animals 26–60 (Plenum Press, 1975). [Google Scholar]
- Rodrigue S. et al. Whole genome amplification and de novo assembly of single bacterial cells. PLoS ONE 4, e6864 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue S. et al. Unlocking short read sequencing for metagenomics. PLoS ONE 5, e11840 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A. E., Mau B. & Perna N. T. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 5, e11147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. & Green P. Consed: a graphical editor for next-generation sequencing. Bioinformatics 29, 2936–2937 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren S. et al. Hybrid error correction and de novo assembly of single-molecule sequencing reads. Nat. Biotechnol. 30, 693–700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W. et al. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systemat. Biol. 59, 307–321 (2010). [DOI] [PubMed] [Google Scholar]
- Kelly L., Huang K. H., Ding H. & Chisholm S. W. ProPortal: a resource for integrated systems biology of Prochlorococcus and its phage. Nucleic Acids Res. 40, D632–D640 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. W., Huang K., Saito M. A. & Chisholm S. W. Transcriptome response of high- and low-light-adapted Prochlorococcus strains to changing iron availability. ISME J. 5, 1580–1594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. J., Johnson Z. I., Szul M. J., Keller M. & Zinser E. R. Dependence of the cyanobacterium Prochlorococcus on hydrogen peroxide scavenging microbes for growth at the ocean's surface. PLoS ONE 6, e16805 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne A. et al. Unraveling the genomic mosaic of a ubiquitous genus of marine cyanobacteria. Genome Biol. 9, R90 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenik B. et al. The genome of a motile marine Synechococcus. Nature 424, 1037–1042 (2003). [DOI] [PubMed] [Google Scholar]
- Palenik B. et al. Genome sequence of Synechococcus CC9311: Insights into adaptation to a coastal environment. Proc. Natl Acad. Sci. 103, 13555–13559 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L., Ding H., Huang K. H., Osburne M. S. & Chisholm S. W. Genetic diversity in cultured and wild marine cyanomyoviruses reveals phosphorus stress as a strong selective agent. ISME J. 7, 1827–1841 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren N. A., Rocap G. & Chisholm S. W. Measurement of Prochlorococcus ecotypes using real-time polymerase chain reaction reveals different abundances of genotypes with similar light physiologies. Environ. Microbiol. 8, 441–454 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Biller S. J. 2014. Prochlorococcus Portal. http://proportal.mit.edu/
- Biller S. J. 2014. Dryad. http://dx.doi.org/10.5061/dryad.k282c
- Biller S. J. 2014. Genbank. JNAG00000000
- Biller S. J. 2014. Genbank. JNAH00000000
- Biller S. J. 2014. Genbank. CP007753
- Biller S. J. 2014. Genbank. JNAI00000000
- Biller S. J. 2014. Genbank. JNAJ00000000
- Biller S. J. 2014. Genbank. JNAK00000000
- Biller S. J. 2014. Genbank. JNAL00000000
- Biller S. J. 2014. Genbank. JNAM00000000
- Biller S. J. 2014. Genbank. JNAN00000000
- Biller S. J. 2014. Genbank. JNAO00000000
- Biller S. J. 2014. Genbank. JNAP00000000
- Biller S. J. 2014. Genbank. JNAQ00000000
- Biller S. J. 2014. Genbank. JNAR00000000
- Biller S. J. 2014. Genbank. JNAS00000000
- Biller S. J. 2014. Genbank. CP007754
- Biller S. J. 2014. Genbank. JNAX00000000
- Biller S. J. 2014. Genbank. JNAT0000000
- Biller S. J. 2014. Genbank. JNAU00000000
- Biller S. J. 2014. Genbank. JNAV00000000
- Biller S. J. 2014. Genbank. JNAW00000000
- Biller S. J. 2014. Genbank. JNAY00000000
- Biller S. J. 2014. Genbank. JNAZ00000000
- Biller S. J. 2014. Genbank. JNBD00000000
- Biller S. J. 2014. Genbank. JNBE00000000
- Biller S. J. 2014. Genbank. JNBA00000000
- Biller S. J. 2014. Genbank. JNBB00000000
- Biller S. J. 2014. Genbank. JNBC00000000